Ephexin1: A Promising Cancer Biomarker?
The latest research on Ephexin1 reveals a promising new twist in our understanding of cancer progression. Traditionally known for its role in the nervous system where it guides axons and maintains synaptic function, recent studies have shown that Ephexin1 is overexpressed in several cancers, notably lung and colon cancers. Its unexpected involvement in regulating protein synthesis makes it an essential culprit behind the rapid growth and spread of tumor cells.
This opinion editorial offers a closer look at the research exploring the tangled issues linking Ephexin1 with cap-dependent translation and mTOR-targeted therapy. The findings raise hope that targeting Ephexin1 may boost therapeutic outcomes, overcome drug resistance, and even lower potential side effects associated with common cancer treatments.
Understanding the Role of Ephexin1 in Protein Synthesis
One of the most compelling aspects of the study is its focus on how Ephexin1 influences cap-dependent translation initiation. Protein synthesis in cancer cells is no simple affair—it involves numerous factors and a series of complicated pieces that must work in concert. In this research, scientists discovered that Ephexin1 interacts directly with translation initiation factors such as eIF2α, eIF3g, and eIF3i. These factors are central to kick-starting the process of translating messenger RNA into proteins.
The study used sophisticated methods such as pulldown assays, co-immunoprecipitation, and polysome profiling to show that Ephexin1 facilitates the assembly of translation initiation complexes. Essentially, when Ephexin1 levels are high, the process that converts genetic information into functional proteins becomes more streamlined, which in turn supercharges the production of proteins that help cancer cells thrive.
Decoding the Cap-Dependent Translation Process
Cap-dependent translation is a form of protein synthesis that starts at the so-called “cap” structure on messenger RNAs. This process is particularly important in cancer cells, where the demand for proteins that promote rapid growth is high. The research indicates that overexpression of Ephexin1 assists in assembling the machinery required at this cap structure—effectively reducing the binding of translation repressors like 4EBP1 and enhancing the interaction of positive factors such as eIF4G and eIF3 subunits.
The study also demonstrated that when Ephexin1 is depleted, many of these initiation factors shift from polysomes (clusters of ribosomes engaged in active translation) to monosomes (individual ribosomes), suggesting a drop in protein synthesis efficiency. This observation helps us understand the finite details outlining how Ephexin1 exactly steers protein production in malignant cells.
mTOR Signaling: A Key Player in Cancer Growth
The mammalian target of rapamycin (mTOR) signaling pathway is known as a central regulator of cell growth and metabolism. Dysregulation of this pathway contributes heavily to cancer progression. In the study, researchers observed that levels of Ephexin1 are correlated with the phosphorylation status of mTOR and its downstream targets, such as S6K1 and 4EBP1, underscoring a vital link between Ephexin1 expression and mTOR-driven processes.
In simple terms, mTOR functions like a master switch in cells, coordinating protein synthesis in response to nutrient availability and growth signals. The finding that Ephexin1 can modulate mTOR activity introduces an exciting possibility: if we can control Ephexin1, we may be able to influence the mTOR pathway, thereby slowing down or even halting cancer growth.
How mTOR Regulation Impacts Cancer Cell Survival
When mTOR is overactive, cancer cells are provided with an excessive amount of proteins and other building blocks that fuel tumor proliferation, invasion, and even metastasis. The current research suggests that higher levels of Ephexin1 enhance mTOR signaling, which in turn maintains an elevated level of translation initiation. This creates a feedback loop that not only spurs cancer progression but also complicates efforts to treat mTOR-driven tumors.
Notably, when mTOR inhibitors such as Torin1 are applied, the effect of reducing cancer cell growth is significantly enhanced in cells that lack Ephexin1. This discovery hints at new ways to combine therapies strategically—a tactic that could improve patient outcomes while potentially lowering the often overwhelming side effects observed with mTOR inhibitors.
Overcoming Drug Resistance with Combined Therapies
Drug resistance remains a scary challenge in oncology. Many cancer treatments, including mTOR inhibitors, often meet with resistance due to alternative pathways that tumors activate to bypass the inhibition. The research brings to light an intriguing possibility: by targeting Ephexin1, we might be able to sensitize cancer cells to mTOR inhibitors, thus breaking down the drug resistance barrier.
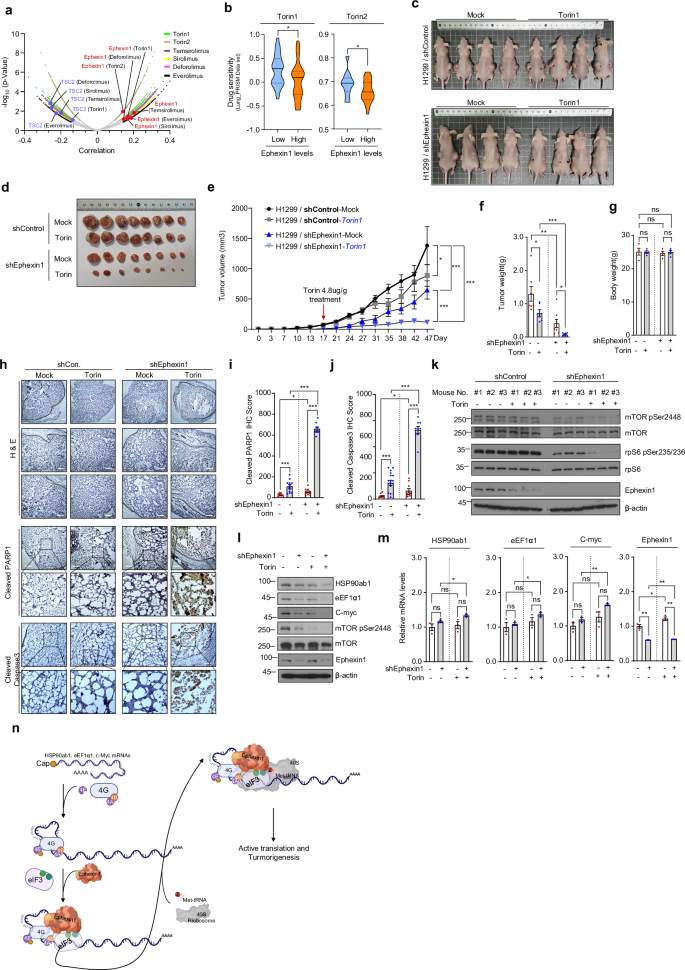
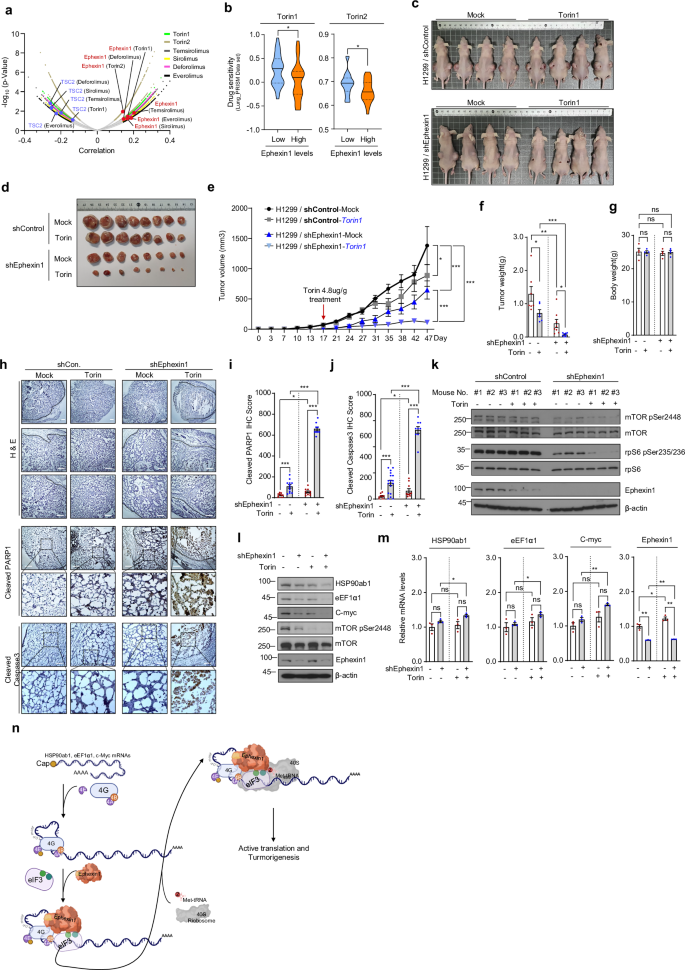
Data from extensive CRISPR screens and drug sensitivity assays indicate a strong positive correlation between reduced Ephexin1 expression and enhanced vulnerability of cancer cells to mTOR-targeted drugs. Conversely, genes that naturally suppress mTOR signaling show a negative correlation with drug sensitivity. This means that in a clinical setting, assessing Ephexin1 levels could help predict which patients are likely to benefit from mTOR inhibitor therapies.
Strategies to Boost mTOR Inhibitor Efficacy
The following bullet list summarizes key strategies that could help enhance the effects of mTOR inhibitors by targeting Ephexin1:
- Combine mTOR inhibitors with Ephexin1-targeting molecules to reduce the overall translation rate in cancer cells.
- Develop diagnostic assays to measure Ephexin1 expression levels in patient tumors, aiding personalized treatment strategies.
- Utilize genetic screening tools to determine which patients harbor high levels of functional Ephexin1, predicting drug response.
- Study the impact of reducing Ephexin1 on other signaling pathways to uncover additional therapeutic targets.
Each of these strategies is designed to weaken the cancer cell’s defense mechanisms and may eventually offer a more efficient, less nerve-racking way to manage treatment resistance.
Ephexin1 and TOP mRNA Translation: Unraveling the Connection
An interesting and somewhat unexpected finding of the study is the connection between Ephexin1 and the selective translation of 5′-TOP mRNAs. TOP mRNAs are a subset of messenger RNAs that contain a specific 5′-terminal oligopyrimidine motif. These mRNAs encode for a variety of proteins that are critical for cell growth, including components of the ribosome and translation machinery.
The research revealed that although ribosomal proteins (typically regulated by TOP motifs) were not heavily altered by changes in Ephexin1 levels, several key cancer-related proteins with TOP or TOP-like motifs, such as HSP90ab1, eEF1α1, c-Myc, and CCT2, were significantly impacted. Specifically, when Ephexin1 was reduced, these proteins showed a marked drop in protein levels without a corresponding decline in their mRNA levels—indicating that the regulation is happening at the translation level.
This observation suggests that Ephexin1 might be fine-tuning the translation of subsets of mRNAs through mechanisms that are still not completely clear but are closely linked to mTORC1 signaling. By modifying the assembly of translation initiation complexes, Ephexin1 seems to target these TOP mRNAs selectively, potentially tipping the scales in favor of cancer cell proliferation.
Implications for Selective Protein Synthesis in Cancer Cells
Selective translation of TOP mRNAs is a critical yet tricky part of cancer cell biology. The fact that Ephexin1 appears to specifically modulate the translation of only certain TOP mRNAs presents a unique therapeutic opportunity. Imagine a scenario where treatment could be fine-tuned to reduce the production of proteins that aid cancer survival, without disrupting the synthesis of proteins essential for normal cell function.
This level of control might also mitigate some of the daunting side effects associated with broad-spectrum mTOR inhibitors, which, while effective, can sometimes cause inhibition of protein synthesis in healthy cells as well.
Clinical Perspectives: Using Ephexin1 as a Prognostic Tool
Beyond its role in translation regulation, Ephexin1 holds promise as a prognostic indicator in the clinic. Immunohistochemistry (IHC) studies on lung cancer tissue samples have revealed that higher levels of Ephexin1, along with elevated expressions of mTOR target proteins like HSP90ab1, c-Myc, and eEF1α, are closely associated with advanced tumor grades and metastasis. This positive correlation reinforces the idea that Ephexin1 is a key driver in tumor development.
What does this mean for patients? Essentially, measuring Ephexin1 levels in tumor biopsies may provide clinicians with a super important biomarker that not only predicts the aggressiveness of the cancer but also hints at the potential efficacy of mTOR-targeted therapies. The integration of such diagnostics into routine clinical practice could help steer treatment decisions, making the process less overwhelming for patients and healthcare providers alike.
Key Points from Clinical Studies
| Biomarker | Associated Outcomes | Implications for Treatment |
|---|---|---|
| Ephexin1 | Elevated expression correlates with higher tumor grade and metastasis | Potential target for sensitizing tumors to mTOR inhibitors |
| HSP90ab1 | Involved in protein stability and stress response | Indicator of active mTOR signaling in cancer cells |
| c-Myc | Key role in cell proliferation and survival | Useful for predicting response to translational inhibitors |
| eEF1α | Regulates protein synthesis chain extension | Serves as a marker for aggressive cancer phenotypes |
This table underscores how intertwined these proteins are in driving cancer progression and hints at how targeting one may impact the entire network.
Addressing the Tricky Parts of Drug Side Effects
One of the most overwhelming challenges in cancer therapy is the management of treatment side effects. mTOR inhibitors, while effective, have been found to cause serious side effects in a significant number of patients. The study suggests that targeting Ephexin1 might allow for lower doses of mTOR inhibitors without sacrificing therapeutic efficacy. This combination strategy could reduce the nerve-racking side effects that patients frequently face.
By selectively reducing the translation of TOP mRNAs specifically associated with cancer progression, therapies that incorporate Ephexin1 targeting could theoretically preserve the essential protein synthesis needed by healthy cells. This distinction is key in improving the quality of life for patients while still combating the tumor aggressively.
Advantages of a Combination Therapy Approach
Combining Ephexin1 inhibition with mTOR-targeted drugs has several potential advantages:
- Lower Required Doses: Reduced amounts of mTOR inhibitors may be needed, lessening toxicity.
- Selective Targeting: By honing in on cancer-specific translation, normal cells remain less affected.
- Improved Efficacy: Enhanced sensitivity of cancer cells to mTOR inhibition may lead to greater tumor shrinkage.
- Reduction in Resistance: Combating multiple molecular pathways concurrently may prevent the tumor from developing alternative survival routes.
Looking Ahead: Combined Therapeutic Strategies in Oncology
The research into Ephexin1 is still in its early stages, but the potential applications are huge. The dual targeting of Ephexin1 and the mTOR pathway could form the basis for a new wave of combined therapies designed to treat aggressive cancers more effectively. Clinical trials in the near future might explore combinations of Ephexin1 inhibitors with established mTOR blockers, testing the hypothesis that such treatments will be more effective and less loaded with side effects than monotherapies.
There is also the exciting possibility of using Ephexin1 levels as a guide for personalized therapy. Not every patient’s tumor will express Ephexin1 at high levels, so tailoring treatment based on a patient’s unique tumor profile might help maximize the benefits while avoiding unnecessary toxicity.
Future Research Directions
To fully harness the potential of Ephexin1 as a target in cancer therapy, further studies are needed to address several challenging bits:
- Uncovering the Fine Points of the Mechanism: Additional research is necessary to dig into how exactly Ephexin1 mediates the assembly of translation initiation complexes and interacts with RNA-binding proteins.
- Developing Specific Inhibitors: Designing molecules that can specifically target Ephexin1 without interfering with its normal functions in the nervous system will be crucial.
- Clinical Trials: Testing combination therapies in well-designed clinical trials will determine whether the laboratory gains translate into real-world benefits for patients.
- Biomarker Validation: Large-scale studies to validate the prognostic value of Ephexin1 and its associated proteins could lead to its inclusion in regular cancer screening protocols.
Tackling the Hidden Complexities of Translational Control
Protein synthesis in cells is like a busy factory with multiple moving parts. The subtle parts of this process involve a series of interactions among various factors to ensure that the right proteins are produced at the right time. Ephexin1 appears to act as a crucial switch in this machinery—from enhancing the shift of translation initiation factors into polysomes to dictating the fate of TOP mRNAs. These hidden complexities form the foundation of how cancer cells manage to thrive even under stressful conditions.
While cell biology is full of twists and turns, the insight that Ephexin1 is deeply involved in these regulatory pathways gives us a clearer direction. It suggests that even the confusing bits of translation machinery might be exploited to gain a therapeutic advantage. With further research, we may soon find ways to figure a path through these tangled issues and identify additional nodes of intervention in the protein synthesis network.
Key Takeaways on Translational Control in Cancer
- Ephexin1 directly binds with critical translation initiation factors, boosting the protein production that cancer cells need.
- The modulation of cap-dependent translation by Ephexin1 is closely linked to the mTOR signaling cascade.
- Selective translation of TOP mRNAs by Ephexin1 provides a new window into how cancer cells prioritize protein synthesis.
- Targeting Ephexin1 may serve as a synthetic lethality strategy, enhancing the effectiveness of mTOR inhibitors and reducing side effects.
The Synthetic Lethality Approach: Ephexin1 as a Therapeutic Target
The concept of synthetic lethality in cancer treatment revolves around targeting a weakness that only cancer cells exhibit due to their unique molecular profile. The research suggests that Ephexin1 is not just a bystander but a key mediator that can be exploited to enhance the effects of mTOR inhibitors. In Ephexin1-deficient cells, even modest doses of drugs like Torin1 lead to significant suppression of tumor growth.
This synthetic lethality approach is particularly attractive because it offers a way to attack the tumor while preserving normal tissue. By combining Ephexin1 inhibition with mTOR-targeted therapy, oncologists may be able to deliver a one-two punch to the cancer, significantly curtailing its ability to proliferate and spread.
Below is a bullet list summarizing why targeting Ephexin1 as part of a synthetic lethality strategy is promising:
- Selective Vulnerability: Cancer cells expressing high levels of Ephexin1 become particularly sensitive to mTOR inhibitors when Ephexin1 is reduced.
- Enhanced Drug Efficacy: Studies show a marked decrease in tumor size and weight in Ephexin1-deficient cells treated with mTOR inhibitors.
- Reduced Side Effects: Lower doses of mTOR inhibitors may be effective when combined with Ephexin1 targeting, potentially reducing treatment-related toxicity.
- Prognostic Value: Monitoring Ephexin1 expression can help predict which tumors are most likely to respond to this combined therapy.
Challenges and Opportunities in Translational Research
Despite these encouraging findings, there remain some tricky parts in the translation of these insights into the clinic. One of the overwhelming challenges involves designing drugs that can specifically target Ephexin1 without affecting its normal functions, particularly in nerve cells where it plays a key role. There is also the daunting task of ensuring that combination therapies do not produce unexpected interactions or adverse effects.
Another layer of complexity is the involvement of several other proteins and signaling molecules that interact with Ephexin1. The translation process incorporates many small distinctions, and even slight differences in the protein synthesis network can have far-reaching effects on cell survival and proliferation. Thus, it is critical to continue pursuing research that dives into the nitty-gritty of these relationships.
Balancing Research and Clinical Needs
For research scientists and clinicians alike, the journey from bench to bedside is full of challenging yet essential steps. Here are some points to consider when balancing experimental insights with the realities of patient care:
- Ensuring that targeted therapies have a low toxicity profile by sparing normal cells.
- Incorporating comprehensive genomic and proteomic analyses to better characterize patient tumors.
- Designing clinical trials that account for the diverse molecular landscapes found within and across tumor types.
- Continually refining our understanding of how Ephexin1 interacts with other pathways, using techniques such as polysome profiling and ribopuromycylation assays.
Final Thoughts: Paving the Way for Future Cancer Treatments
The research on Ephexin1 and its role in regulating cap-dependent translation through mTOR signaling opens intriguing new avenues for cancer treatment. By diving into the fine points of how Ephexin1 promotes the translation of specific, cancer-driving genes, scientists are uncovering a potential weak spot in the robust machinery of cancer cells.
The evidence suggests that combining Ephexin1-targeted therapies with existing mTOR inhibitors could lead to improved outcomes. Such a combination could not only amplify the drugs’ effectiveness but also help arrow-straight a path through some of the nerve-racking side effects commonly associated with mTOR inhibitors.
While the road ahead is loaded with issues and tricky parts—designing specific inhibitors, deciphering the subtle details of the initiation process, and ensuring safe clinical application—the potential rewards are enormous. Personalized medicine, guided by biomarkers such as Ephexin1, could transform the way we approach treatment for aggressive cancers.
Over the long term, this research highlights the need for a more integrated approach in oncology—one that picks apart the confusing bits of cellular processes, figures a path through the tangled issues of drug resistance, and ultimately leads to treatments that are both effective and gentle on patients. As we continue to poke around the hidden complexities of cancer cell biology, Ephexin1 stands out as a promising target that could lead to smarter therapies tailored to the specific molecular profiles of tumors.
Summary of Key Points
- Ephexin1 Overexpression: Emerging evidence indicates that high levels of Ephexin1 drive cancer progression by promoting cap-dependent translation.
- Direct Interaction with Translational Machinery: Ephexin1 directly binds with translation initiation factors, which enhances the production of proteins vital for tumor growth.
- mTOR Pathway Activation: There is a strong correlation between Ephexin1 levels and mTOR signaling, suggesting that it plays a critical role in mTOR-mediated translational control.
- Selective Regulation of TOP mRNAs: Ephexin1 appears to selectively influence translation of proteins coded by TOP mRNAs, many of which are key players in cell proliferation.
- Enhanced Sensitivity to Inhibitors: Reduction of Ephexin1 makes cancer cells more sensitive to mTOR inhibitors, offering a new potential strategy to overcome drug resistance.
- Clinical Implications: Ephexin1 and its related proteins serve as promising biomarkers for assessing tumor aggressiveness and could aid in designing personalized treatment protocols.
Concluding Remarks
If there is one clear takeaway from the research on Ephexin1, it is that the integration of basic molecular insights with clinical strategies holds enormous potential for the future of cancer treatment. The tangled issues surrounding protein synthesis in cancer cells are gradually being unraveled through studies like these, which take a closer look at the roles of individual players like Ephexin1.
Moving forward, both the scientific community and clinicians will need to work together to translate these findings into effective treatment regimens. With continued investment in understanding the small distinctions of translation regulation and cell signaling, researchers can pave the way for combination therapies that are both super important for curbing cancer progression and designed to limit adverse effects.
As we make our way through the twists and turns of cancer biology, the ability to work through and manage these complex, often intimidating processes is a testament to the progress in modern medicine. Ephexin1 serves not only as a promising target for synthetic lethality in mTOR-driven cancers but also as a symbol of how incremental insights can eventually lead to significant leaps forward in patient care.
This pioneering research reminds us that despite the nerve-racking challenges ahead, the continued pursuit of understanding and overcoming the hidden complexities of cancer cell biology is our best hope for a future where cancer is a manageable condition rather than an insurmountable threat.
Originally Post From https://www.nature.com/articles/s12276-025-01520-2
Read more about this topic at
The role of Ephexin1 in translation and mTOR-targeted …
Ephexin1 Is Required for Eph-Mediated Limb Trajectory …