Epigenetic Roadblocks in Lung Cancer: Rethinking Cisplatin Resistance
Lung cancer remains a leading cause of cancer-related deaths around the globe. Despite advances in chemotherapy, particularly through agents such as cisplatin, the overall survival rate barely improves. A chief challenge is the emergence of cisplatin resistance, which seriously undermines treatment efficacy. In this editorial, we take a closer look at how epigenetic regulation—not just genetic mutations—plays a critical role in shaping this resistance, and we discuss how new advancements and targeted therapies might help find a path forward.
Understanding the Molecular Approach: How Cisplatin Fights Cancer
Cisplatin works by directly damaging the DNA in tumor cells, triggering cell death through either apoptosis or necrosis. On a molecular level, the drug binds to DNA, creating crosslinks that obstruct replication. This inhibition sets off several key signaling pathways, including those involving ATR, p53, and MAPK, eventually pushing cells over the edge to programmed death. However, as treatment progresses, cancer cells often develop clever mechanisms to repair this damage or bypass these death signals, resulting in what can only be described as a tangled issue in therapy.
The drug’s action is further complicated by the fact that cisplatin also induces oxidative stress via reactive oxygen species (ROS). These reactive molecules add another layer to the already complicated pieces of drug resistance. Thus, while cisplatin remains a cornerstone for treating several types of solid tumors, its long-term effectiveness is too often limited by the cancer cells’ ability to counter these effects.
DNA Methylation and Its Key Role in Drug Resistance
Tangled Issues with Gene Silencing
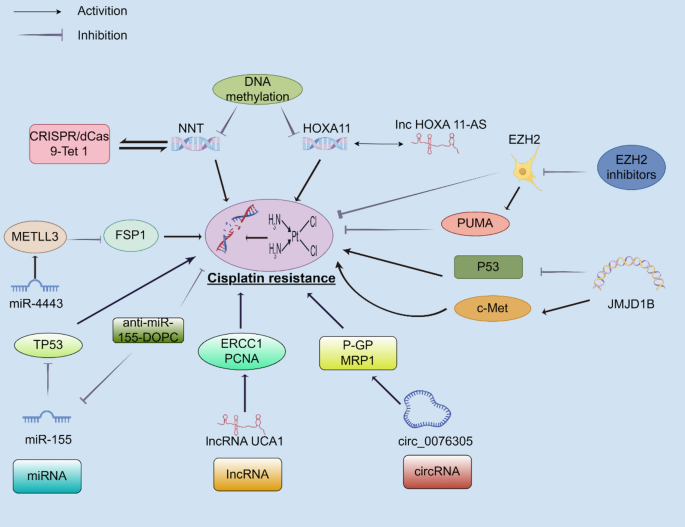
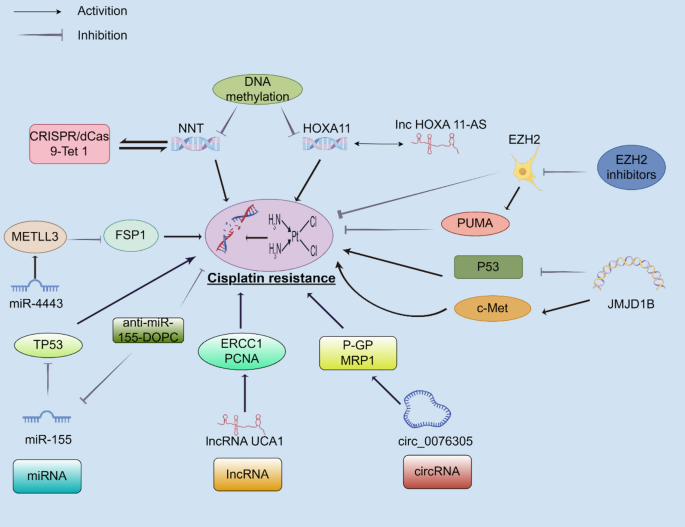
One of the key epigenetic mechanisms involved in cisplatin resistance is DNA methylation. This process, which involves the addition of a methyl group to cytosine bases in DNA, usually results in gene silencing when present at gene promoters. In lung cancer, hypermethylation can shut down tumor suppressor genes, thereby giving cancer cells an edge to survive under chemotherapeutic stress. For example, genes like NNT and HOXA11 may be turned off by increased methylation, promoting survival mechanisms such as autophagy and reduced apoptosis.
When these tumor guard proteins are silenced, the cells may activate their survival and repair processes more efficiently—even when faced with cisplatin-induced DNA damage. Researchers have even observed that lung cancer tissues with higher levels of promoter hypermethylation tend to be more resistant to treatment. This discovery highlights the urgent need to consider epigenetic status—not just genetic mutations—in devising treatment strategies.
Table: Impact of DNA Methylation on Key Genes
| Gene | Normal Role | Effect of Hypermethylation |
|---|---|---|
| NNT | Regulates cellular redox balance and supports apoptosis | Silencing leads to enhanced autophagy and drug resistance |
| HOXA11 | Functions as a tumor suppressor | Downregulation promotes epithelial–mesenchymal transition (EMT) and resistance |
| FOXF1 | Regulates cell differentiation | Dysregulation contributes to altered cell cycle control and resistance |
The table above illustrates how hypermethylation of crucial genes can lead to their silencing and thereby fuel cisplatin resistance. This epigenetic twist not only adds to the drug’s diminished effectiveness but also underscores the importance of reversing these modifications as a therapeutic strategy.
Noncoding RNAs: The Small Distinctions That Make a Big Difference
MicroRNAs and Their Role in Steering Drug Sensitivity
Noncoding RNAs—especially microRNAs (miRNAs)—are emerging as critical regulators of how cancer cells respond to cisplatin. These small RNA fragments, typically 19–25 nucleotides long, work by binding to target messenger RNAs and thus hindering their translation into proteins. Some miRNAs can reduce the expression of genes that are usually involved in DNA repair and apoptosis, making the cells more sensitive to the drug. Conversely, others may act to block apoptosis, thereby encouraging resistance.
For instance, miR-155 is often found in greater amounts in cisplatin-resistant cells, where its high expression can inhibit p53, a critical tumor suppressor protein. This creates a scenario in which the cisplatin-induced death signals become considerably weaker. With cancer cells using these tiny molecules as both shield and sword, targeting miRNAs emerges as a super important strategy to overcome resistance.
Long Noncoding RNAs: The More Extensive Players
Long noncoding RNAs (lncRNAs) are another group of noncoding transcripts that exceed 200 nucleotides. These RNAs have a variety of roles, including serving as molecular sponges that bind miRNAs, thereby modulating gene expression indirectly. LncRNAs such as UCA1, MALAT1, and HOXA-AS3 have been implicated in promoting cisplatin resistance. They achieve this by enhancing DNA repair pathways, altering cell cycle control, and modulating cell death signals.
By raising the levels of certain lncRNAs in resistant cells, it appears that the tumor microenvironment becomes more inclined to shield itself against the full force of cisplatin. The subtle parts of their regulation suggest that even minor fluctuations in the expression of these lncRNAs can have a dramatic impact on chemotherapy outcomes.
Circular RNAs: The Hidden Complexities Behind Resistance
Circular RNAs (circRNAs) are another layer of RNA regulation in which the molecules form a closed loop with no free ends, offering them a unique stability. Some circRNAs, such as circPVT1, have been shown to act as a sponge for miR-145-5p. By doing so, they indirectly maintain the high expression of proteins such as ABCC1—a key transporter involved in drug efflux.
This means that when circRNAs are overexpressed, they can help the resistant cells pump out the chemotherapy drug more effectively, lowering its intracellular concentration and weakening its lethal effect. On the flip side, downregulated circRNAs may lead to loss of certain tumor suppressor activities, further rounding out the many twisted ways in which noncoding RNAs can affect drug sensitivity. This multifaceted regulation is full of problems yet also offers multiple avenues for potential intervention.
Histone Modifications: Fine Shades of Regulation in Drug Resistance
The Role of Histone Methylation in Silencing Genes
Another key mechanism that influences the responsiveness to cisplatin is histone modification, particularly methylation. Histones are proteins that package DNA and help regulate gene expression by controlling how tightly DNA is wound. Changes in histone methylation and acetylation can either block or encourage the transcription of critical genes involved in cancer cell survival.
For example, an enzyme called EZH2 is responsible for methylating histone H3 on lysine 27. When overexpressed, EZH2 can silence the expression of pro-apoptotic genes such as PUMA, leading to enhanced cell survival in the face of cisplatin treatment. This fine point of regulation adds another layer of complexity to how lung cancers manage to dodge the full impact of chemotherapy.
Histone Acetylation and Its Impact on Gene Expression
Histone acetylation, another modification, generally has the opposite effect: it relaxes the chromatin structure, facilitating gene transcription. Histone deacetylases (HDACs) remove these acetyl groups, potentially leading to the suppression of genes that are needed for effective drug-induced apoptosis. In fact, HDAC inhibitors have shown promise in restoring the sensitivity of lung cancer cells to cisplatin.
One promising approach is the combination of HDAC inhibitors with cisplatin, which can help re-establish a pro-apoptotic environment even in cells that have managed to fortify their defenses through epigenetic means. The small distinctions in histone modifications represent not merely a side note but a key battleground in the fight against cisplatin resistance.
M6A RNA Methylation: Emerging Trends in Epigenetic Regulation
N6-methyladenosine (m6A) RNA methylation is one of the more recently recognized and widely distributed RNA modifications. This specific change is administered by the METTL3/METTL14 complex and plays an essential part in regulating RNA stability and translation efficiency. In lung cancer cells, aberrant m6A modifications can adjust the translation of mRNAs that encode proteins involved in drug resistance.
For instance, increased m6A modification on YAP mRNA—a key regulator in cell proliferation and survival—triggers its higher protein synthesis in resistant cells. Not only does this modification help the cells dodge cisplatin’s effects, but it also promotes migration and metastasis. The emerging trend here is that by targeting m6A modifications, either through the use of specific inhibitors or by employing RNA editing methods, we may be able to tilt the scales back toward drug sensitivity.
An intriguing possibility is that interference with m6A ‘readers’—the proteins that bind to these methylated RNAs and facilitate their function—might undo some of the resistance mechanisms. In this way, targeting m6A modifications offers a promising route for future research and therapeutic development.
Challenges in Overcoming Resistance: The Intimidating Hurdles
Unraveling the Complicated Pieces of Resistance
While we have made significant strides in understanding how epigenetic modifications influence cisplatin resistance, the tangled issues remain vast and layered. The mechanisms are not isolated; instead, they interfere with and complement one another. For instance, DNA methylation, histone modifications, and noncoding RNA regulation all intersect to influence gene expression, DNA repair, cell cycle control, and apoptosis. This overlapping network creates a nerve-racking scenario where even small changes in one part of the system can cascade into major shifts in drug sensitivity.
In real-world clinical settings, these epigenetic changes often vary from patient to patient, making it challenging to formulate a one-size-fits-all treatment approach. Heterogeneity in lung cancer means that the epigenetic status of one tumor may be vastly different from another’s, despite similar histological classifications. Such subtle parts of regulation, the little details that we sometimes overlook, can have huge ramifications for how patients respond to therapy.
Resistance as a Multifactorial and Evolving Process
Cisplatin resistance is not a static trait. Instead, it evolves over time, particularly under the selective pressure of chemotherapy. Initially, some tumors are intrinsically resistant while others develop resistance as the treatment progresses. This evolution is driven by multiple factors, including the upregulation of drug efflux pumps, activation of survival signaling pathways, and stimulated DNA repair mechanisms. The process is full of issues, and the rate at which resistance emerges can vary widely between patients.
To address these challenges, a deeper understanding and constant monitoring of the epigenetic landscape is required. Liquid biopsy techniques, which can sample circulating tumor cells or cell‐free DNA, offer a non-invasive way to keep an eye on these changes over time. Such approaches could help clinicians figure a path through the messy epigenetic terrain, tailoring interventions as resistance evolves.
Clinical Applications: Sorting Out Epigenetic Therapy
Combination Therapies: A Promising Strategy
One of the most promising strategies to overcome cisplatin resistance is to combine conventional chemotherapy with epigenetic modifiers. DNA methyltransferase inhibitors (DNMTis) such as decitabine and azacitidine, as well as HDAC inhibitors like vorinostat, have shown potential in preclinical studies. When used alongside cisplatin, these agents can restore the expression of tumor suppressor genes and re-sensitize cancer cells. Although these drugs come with their own set of challenges—ranging from bone marrow suppression to gastrointestinal discomfort—their ability to rewire the cancer cell’s epigenetic program is too significant to ignore.
Clinical Trials and Ongoing Research
Several clinical trials have already explored the combination of epigenetic drugs with chemotherapy. For example, trials combining HDAC inhibitors with cisplatin-based regimens have demonstrated higher response rates, although the improvements in overall survival remain modest. The complexity of these treatments, along with their potential side effects, makes it imperative that we refine dosing strategies and patient selection criteria. It is essential to identify which patients are most likely to benefit from such combinations, so that we can steer through the overwhelming clinical challenges with precision.
Moreover, recent advancements in CRISPR-based epigenetic editing technologies offer new promise. Tools such as CRISPR/dCas9 fused with epigenetic modifiers can precisely target specific genes for demethylation or methylation, offering a more personalized and potentially less toxic approach. Imagine being able to directly reprogram a resistant tumor cell back to a state where it is once again sensitive to cisplatin—that’s the promise of these innovative techniques.
Table: Examples of Epigenetic Treatments in Clinical Settings
| Epigenetic Modifier | Target Pathway | Potential Benefit | Challenges |
|---|---|---|---|
| Decitabine/Azacitidine | DNA Methylation | Restores tumor suppressor gene expression | Bone marrow suppression, dosing difficulties |
| Vorinostat (HDACi) | Histone Acetylation | Enhances chemo-induced apoptosis | Gastrointestinal issues, dose-related toxicities |
| CRISPR/dCas9-Epigenetic Editors | Precise gene regulation | Personalized intervention, re-sensitization | Delivery methods, off-target effects |
This table underscores both the potential and the challenges of integrating epigenetic modifiers into treatment regimens. It is clear that while the science is promising, successful clinical application requires careful fine-tuning and patient-specific strategies.
Strategies to Tackle Epigenetic Resistance: Finding a Path Forward
Addressing the Intimidating and Overwhelming Challenges
Given the nerve-racking complexities surrounding cisplatin resistance, what should be our game plan? First, it is critical to employ a more holistic approach when designing treatment regimens. Rather than treating the resistance mechanisms as isolated events, clinicians should consider the entire network of epigenetic changes—be it DNA methylation, histone modifications, noncoding RNA expression, or m6A methylation. By examining the complete picture, we can start to identify common pathways and potential synergies between various treatment modalities.
Another key approach is developing reliable biomarkers. With the help of non-invasive techniques such as liquid biopsies, it is possible to track the evolution of these epigenetic markers over time. Reliable biomarkers can guide clinicians in choosing when to introduce epigenetic therapies, how to tailor dosing, and even when to switch strategies if resistance begins to mount. These monitoring tools are super important for managing the long and winding process of chemotherapy resistance.
Role of Personalized Medicine in Overcoming Resistance
One of the most exciting prospects in this field is personalized medicine. By combining next-generation sequencing with advanced epigenetic profiling, we can better understand the specific epigenetic signature of an individual’s tumor. This detailed profiling allows healthcare providers to make informed decisions about targeted therapies, drastically increasing the chance of reversing cisplatin resistance.
Personalized interventions might include, for example, using CRISPR-based editing tools to demethylate specific gene promoters in tumors that show hypermethylation-associated resistance. In other cases, the treatment plan might revolve around modulating the levels of certain miRNAs that are known to impact chemotherapy sensitivity. The ultimate aim is not one-size-fits-all, but rather a tailored approach that takes into account the little twists and turns of each patient’s disease.
The Future: Emerging Technologies and Epigenetic Editing
CRISPR-Based Epigenetic Editing Tools
A promising beacon on the horizon is the advent of CRISPR-based epigenetic editing tools. Instead of introducing broad-spectrum drugs that affect multiple genes at once—and sometimes cause toxic side effects—these tools allow for precise reprogramming of epigenetic modifications. For example, the CRISPR/dCas9-TET1 system can selectively demethylate the promoters of key genes, thereby reactivating them and restoring drug sensitivity. This is not just an interesting scientific finding; it represents a tangible step toward overcoming the overwhelming challenges posed by cisplatin resistance.
Such precision tools hold great promise for the future because they can be designed to target exactly the subtle details of a tumor’s epigenetic makeup. Moreover, by understanding and addressing the fine shades of gene regulation, we stand a better chance of counteracting the cancer cells’ evasive maneuvers.
Delivery Mechanisms and Safety Concerns
Nevertheless, the road to clinical application is loaded with challenges. One of the toughest obstacles is ensuring that these novel therapies can be delivered safely and effectively. The delivery mechanisms—from viral vectors to lipid nanoparticles—must be refined to avoid off-target effects and immune responses. Another challenge is managing the dose—too little might be ineffective, but too much could create additional complications. These nerve-wracking issues underscore the need for rigorous clinical trials and careful optimization.
Early clinical results are encouraging, but the complexity of these treatments means that their effects can be unpredictable. As researchers and clinicians continue to refine these approaches, it is important to remember that even small improvements in targeting the correct epigenetic modifications can have a huge impact on long-term outcomes.
Balancing Risks and Rewards in Epigenetic Therapies
Cost, Toxicity, and Individual Variability
While the promise of epigenetic therapies is immense, they are not without risks. Many of the available epigenetic drugs have dose-related toxicities that can be overwhelming for patients—issues like bone marrow suppression, liver impairment, or gastrointestinal discomfort. Furthermore, the individual variability in patients’ tumors means that what works for one may not work for another. This variability is a direct result of the myriad tangled issues in epigenetic regulation and tumor heterogeneity.
To manage these challenges, doctors need to carefully balance the potential benefits of epigenetic therapies with their side effects. Personalized diagnostic tools and better monitoring can help steer through this charged landscape. The development of more refined delivery methods, alongside improved biomarkers, will be key measures in making these treatments safer and more effective for a broader population of patients.
Regulatory and Logistical Hurdles
The integration of epigenetic therapies into everyday clinical practice is also loaded with regulatory and logistical concerns. Since these treatments blend the complex techniques of gene editing with standard chemotherapy, they often fall under strict regulatory scrutiny. In addition, the cost of developing and administering such advanced therapies may be high, potentially limiting accessibility for many patients.
Despite the challenges, the need for innovative solutions in overcoming cisplatin resistance is undeniable. By combining conventional therapies with emerging epigenetic interventions, we can ultimately optimize treatment outcomes. Stakeholders must work closely with regulatory authorities to streamline the approval process while ensuring patient safety and treatment efficacy.
Conclusion: Embracing the Future of Epigenetic Therapies in Lung Cancer
The battle against lung cancer is full of problems—tangled issues that require extra care and innovative thinking to overcome. As we take a closer look at the role of epigenetic modifications in cisplatin resistance, it becomes clear that a multi-pronged approach is essential. Whether it is by targeting DNA methylation, adjusting histone modifications, modulating noncoding RNAs, or exploring m6A RNA methylation, each of these pathways offers potential routes to re-sensitize tumors to chemotherapy.
Combination therapies that incorporate epigenetic drugs with conventional chemotherapy represent a super important strategy in the fight against drug resistance. With the aid of personalized medicine and emerging CRISPR-based epigenetic editing tools, we have the chance to tailor treatments to the unique profile of each patient’s tumor, thereby improving their overall outcomes.
Although there remain many intimidating challenges—ranging from unpredictable toxicities to the nerve-racking heterogeneity of lung tumors—the future is bright. Ongoing research and clinical trials are continually refining our understanding of these subtle parts of gene regulation. The hope is that by further understanding these complicated pieces, we can finally figure a path through the maze of resistance and offer more effective treatments to lung cancer patients worldwide.
In summary, the adoption of epigenetic strategies marks an essential turning point in lung cancer therapy. As basic research continues to expose the little twists and subtle details of this regulatory machinery, and clinical trials demonstrate the potential of these new treatments, we are increasingly optimistic about our ability to overcome one of the most overwhelming obstacles in cancer therapy: cisplatin resistance.
We now stand at the frontier of a new era in cancer treatment—a time when the interplay between classical chemotherapy and cutting-edge epigenetic modifications may transform the landscape of lung cancer care. While the road ahead is loaded with issues and daunting challenges, the progress made thus far inspires hope, suggesting that the integration of these innovative approaches could turn the tide against this deadly disease.
Originally Post From https://clinicalepigeneticsjournal.biomedcentral.com/articles/10.1186/s13148-025-01961-6
Read more about this topic at
Epigenetic alterations and mechanisms that drive …
Targeting epigenetic regulators to overcome drug … – Nature