Astrocyte Signaling and Blood–Brain Barrier Disruption: A Closer Look
The recent research on intracerebral hemorrhage (ICH) and its impact on the blood–brain barrier (BBB) has opened new avenues for understanding the tricky parts of neurovascular injury. In ICH—a type of stroke notorious for its overwhelming neurological deficits—a cascade of inflammatory events unfolds as the BBB is compromised. The study in question examines the role of astrocyte-derived CXCL10 and its interaction with the CXCR3 receptor on endothelial cells. It also brings to light the importance of the cGAS-STING and AIM2 pathways in promoting pyroptosis (a type of programmed cell death) that further disrupts the BBB. This opinion editorial will take a closer look at these findings, dig into the little details behind these intricate processes, and discuss how they might shape the future of ICH therapies.
In this piece, we will figure a path through the following topics:
- Understanding BBB dysfunction in intracerebral hemorrhage
- The role of astrocyte-derived CXCL10 in vascular pyroptosis
- Selective CXCR3 inhibition as a promising therapeutic approach
- The interplay between cGAS-STING and AIM2 inflammasomes in endothelial cell injury
- Potential benefits and concerns regarding future ICH treatments
- Integrating cellular crosstalk in neuroinflammatory responses
Understanding BBB Dysfunction in Intracerebral Hemorrhage
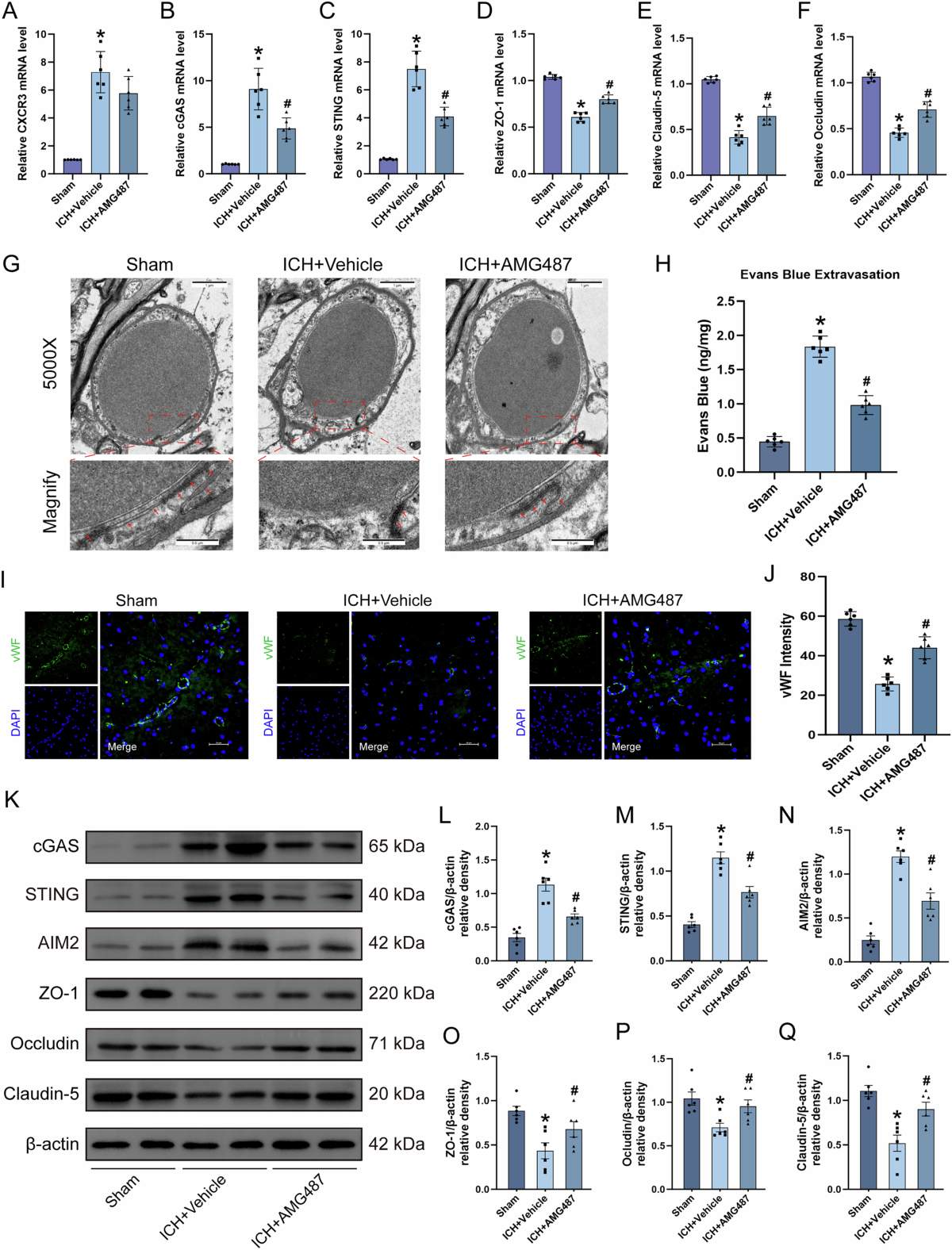
Intracerebral hemorrhage accounts for a significant percentage of stroke cases, and its aftermath is riddled with numerous challenges. The BBB, which acts as a critical defense line between the central nervous system and the peripheral circulation, suffers extensive damage during ICH. This damage, marked by the breakdown of tight junction proteins such as ZO-1, occludin, and claudin-5, results in increased brain water content (edema) and compromises proper neurological functioning.
When evaluating the BBB in the context of ICH, the research underscores several tangled issues, including:
- The degradation of the tight junctions that normally maintain BBB integrity.
- The release of damaging agents from the blood and inflammatory cells that infiltrate the brain tissue.
- The involvement of various signaling pathways that intensify the inflammatory response.
Addressing these confusing bits requires not only an understanding of the fine points of BBB anatomy but also a deep dive into how inflammatory cascades are triggered. The disturbance in the BBB is much more than a mere physical leak; it is a marker of a multifaceted inflammatory process that can lead to substantial neurological deficits post-ICH. The interplay between cellular and molecular actors, such as astrocytes and cytokines, reveals the importance of maintaining the barrier as a critical—and super important—step in preventing further brain damage.
Astrocyte-Derived CXCL10 and Its Role in Endothelial Pyroptosis
A key aspect of the study is the focus on CXCL10, a molecule secreted by astrocytes and endothelial cells, which acts as an activating ligand for the CXCR3 receptor. CXCL10 is upregulated in the acute phase of ICH and is closely linked to poor outcomes in patients. Its increased expression serves as an essential signal that sets off a series of changes leading to endothelial pyroptosis.
Endothelial cells, which line the blood vessels of the brain, are directly impacted by CXCL10. When CXCL10 binds to CXCR3 on these cells, it not only contributes to the degradation of tight junction proteins but also triggers the activation of inflammatory cascades through the cGAS-STING and AIM2 signaling pathways. The resulting pyroptosis contributes to further BBB disruption, which can exacerbate edema and neurological impairments.
Some critical points regarding the role of astrocyte-derived CXCL10 include:
- Its time-dependent upregulation following ICH, peaking around 24 hours post-event.
- The localization of CXCL10 primarily to cerebrovascular endothelial cells and astrocytes, indicating a dual-origin that amplifies its effect.
- The ability of exogenous CXCL10 administration to worsen neurological deficits and promote cerebral edema, thus providing evidence of its key role in inflammatory amplification.
By taking a closer look at these interactions, we understand that the astrocyte’s role extends far beyond mere support. Instead, astrocytes are active participants in the tangled cascade of neuroinflammation and BBB compromise that characterizes ICH.
Selective CXCR3 Inhibition: A Key Therapeutic Strategy
The potential of selective CXCR3 inhibition as a therapeutic strategy in ICH has emerged as one of the most promising findings. In the study, the use of AMG487, a selective CXCR3 inhibitor, was shown to improve neurological function by mitigating the disruption of the BBB and reducing cerebral edema. AMG487 was administered in a range of doses, and the optimal dosage was determined by improvements in behavioral tests and reductions in brain water content.
Key findings that support CXCR3 inhibition as a practical therapeutic approach include:
- A reduction in the levels of inflammatory markers (cGAS, STING, AIM2) which are typically elevated following ICH.
- Restoration of tight junction proteins that help to re-establish the BBB’s structural integrity.
- Improvement in neurological outcomes, as measured by modified Garcia tests and other behavioral assessments.
By steering through the tangled issues of neuroinflammation, CXCR3 inhibition via AMG487 appears to block the downstream activation of the cGAS-STING/AIM2 pathway. In doing so, it preserves the architectural and functional aspects of the BBB, which is super important for recovering from ICH and preventing further brain damage.
Interplay Between cGAS-STING and AIM2 Inflammasomes: The Nitty-Gritty of Endothelial Cell Injury
The study places considerable emphasis on the cGAS-STING and AIM2 signaling pathways, meticulously examining their roles in driving endothelial pyroptosis after ICH. When the BBB is compromised, the release of endogenous double-stranded DNA (dsDNA) into the cytoplasm activates the enzyme cGAS. This enzyme then catalyzes the production of cyclic GMP-AMP (cGAMP), which engages with STING to promote inflammatory signaling.
This chain reaction further involves AIM2—a sensor that recognizes dsDNA and triggers pyroptosis by assembling inflammasomes. The resulting release of inflammatory cytokines such as IL-1β and IL-18 further deepens the damage at the BBB level.
The study highlights several small distinctions in the way these pathways interact:
- Activation of cGAS in brain endothelial cells triggers an inflammatory cascade that promotes cell death.
- The subsequent activation of STING and AIM2 contributes to the assembly of the inflammasome complex, amplifying the release of pro-inflammatory molecules.
- Both genetic inhibition (using siRNA) and pharmacological suppression (using the dual antagonist A151) of these pathways have shown potential in reducing BBB deterioration.
In essence, these findings shed light on the fine details of how endothelial cells succumb to inflammatory stress post-ICH. By blocking or dampening these pathways, researchers hope to stop the feedforward cycle of inflammation and BBB breakdown, thus offering a window for therapeutic intervention.
Crosstalk Between Endothelial Cells and Astrocytes: Managing Your Way Through Inflammatory Responses
Another intriguing aspect of this research is the exploration of the cellular crosstalk between endothelial cells and astrocytes. The BBB is not maintained by a single cell type; rather, it is the result of collaborative interactions between different cells, including astrocytes, pericytes, and endothelial cells. Astrocytes, in particular, play a critical role in releasing CXCL10 and other inflammatory mediators.
The study used both monoculture and co-culture systems to simulate the in vivo effects of CXCL10. In vitro models revealed that the neutralization of CXCL10 reduced endothelial pyroptosis and downregulated the expression of key inflammatory proteins. These findings suggest that the communication between endothelial cells and astrocytes is loaded with issues that exacerbate BBB damage.
Some thoughts on this matter include:
- The co-culture system mirrors the in vivo environment more accurately than endothelial cell monoculture, capturing the multiple layers of inflammatory crosstalk.
- Neutralizing CXCL10 in such systems demonstrated a measurable decrease in the activation of the cGAS-STING/AIM2 pathway, thereby providing a practical focal point for future drug targeting.
- This cellular interplay adds another layer of complexity—yet it also offers additional therapeutic targets, such as specific cytokine neutralizers or signaling inhibitors.
Taking a closer look at the interactions between these cell types reveals not only the challenges of mitigating neuroinflammation but also the potential avenues where intervention might prove effective. Such multidisciplinary approaches could be the key to managing the feedforward cycles seen in severe ICH cases.
Potential Benefits and Concerns for Future ICH Treatments
When evaluating any new therapeutic approach, it is crucial to consider both its promising benefits and the off-putting hurdles that might stand in the way of clinical application. The idea of targeting the CXCR3 pathway, in particular, holds significant promise given the study’s findings. AMG487, for example, has demonstrated the ability to reduce neurological deficits, improve BBB integrity, and downregulate key inflammatory pathways.
However, there are several tricky parts and challenging bits to keep in mind:
- Drug Delivery Challenges: AMG487, with its relatively high molecular weight, exhibits limited BBB penetrability. As a result, researchers had to use intracerebroventricular administration to achieve therapeutic levels. Finding a less invasive and more systemic delivery route remains a nerve-racking task.
- Gender Differences in Response: The study opted for male mice to avoid the confounding influence of estrogen fluctuations. However, this raises the question of whether similar results would be observed in female subjects, whose inflammatory responses might differ due to hormonal variations.
- Complexity of Signaling Networks: The cGAS-STING/AIM2 axis is not the only inflammatory pathway at work in ICH. Inflammatory responses in the brain are often full of problems and are compounded by the involvement of microglia, neurons, and peripheral immune cells. This layered complexity means that a single-target approach might not be sufficient.
Despite these concerns, the study offers a hopeful prospect. By demonstrating that the suppression of specific inflammatory pathways can alleviate BBB disruption, it provides a solid foundation on which future therapies might be developed. In the big picture, success will likely depend on multi-target strategies that can address the various twists and turns of post-ICH inflammation.
Key Insights on Integrating Inflammatory and Neuroprotective Strategies
One of the most engaging aspects of current research is its holistic take on the neurovascular unit. Rather than viewing the BBB as a simple barrier, cutting-edge studies now regard it as a dynamic structure impacted by a variety of cellular interactions and inflammatory signals. Integrating our understanding of both the pro-inflammatory and neuroprotective pathways can be crucial in developing effective treatments.
This comprehensive approach suggests a few strategic directions:
- Combining Drug Therapies: A combination of CXCR3 inhibitors, like AMG487, with cGAS-STING/AIM2 pathway blockers, such as A151, may provide a more robust defense against BBB disruption.
- Enhancing Tight Junction Stability: Future treatments might focus on enhancing the expression or function of tight junction proteins directly, helping to maintain or restore BBB integrity even in the face of inflammatory assault.
- Targeting Multiple Cell Types: Since multiple cell types contribute to the overall dysfunction of the BBB, therapeutic strategies that simultaneously modulate astrocytic activity, microglial response, and endothelial cell survival might prove more effective.
Below is a table summarizing some of the critical factors at play:
| Component | Role in ICH | Potential Target |
|---|---|---|
| CXCL10 | Upregulated in astrocytes and endothelial cells; triggers CXCR3 | Neutralizing antibodies or inhibitors |
| CXCR3 | Receptor on endothelial cells; mediates inflammatory signaling and pyroptosis | Selective inhibitors like AMG487 |
| cGAS-STING | Detects cytosolic dsDNA, initiating inflammatory cascades | Pharmacological inhibitors and siRNA approaches |
| AIM2 Inflammasome | Assembles in response to dsDNA, promoting pyroptosis and cytokine release | Dual antagonists such as A151 |
| Tight Junction Proteins | Maintain the integrity of the BBB | Therapies aimed at preserving or restoring protein expression (ZO-1, occludin, claudin-5) |
This table highlights the critical interplay between these factors and emphasizes the need for multifaceted therapeutic approaches to properly address the full scope of BBB disruption during ICH.
Digging into the Cellular Mechanisms: Scientific and Clinical Implications
One of the major leaps in the research is the detailed examination of the mechanism behind endothelial pyroptosis. By using both in vivo and in vitro models, the study shows how exogenous stimulation (through agents like CXCL10/IP-10) can sharpen the inflammatory response. The sequence is as follows:
- ICH initiates the release of damage-associated molecular patterns (DAMPs), such as dsDNA, into the cellular environment.
- This dsDNA activates cGAS, which then produces cGAMP, further stimulating the STING pathway.
- The activation of these pathways leads to the assembly of the AIM2 inflammasome, which in turn triggers pyroptosis in endothelial cells.
- The continuing cycle exacerbates the degradation of tight junctions, ultimately worsening the BBB breakdown.
The schematic diagram included in the study (though not visible here) clearly maps out these steps. The clinical implication is that each step represents a potential point at which compromised inflammatory responses could be intercepted, thereby providing layered protection to the BBB. Such detailed mapping of signaling events is critical when designing combination therapies that target several nodes simultaneously rather than relying on a single intervention.
Comparing Inhibitory Strategies: AMG487 Versus Broad-Spectrum Approaches
Not all treatments are created equal. One of the striking advantages of selective CXCR3 inhibition is its ability to precisely target the receptors implicated in endothelial injury without broadly dampening the entire immune response. While broad-spectrum anti-inflammatory drugs often leave patients susceptible to infections or other unintended side effects, AMG487’s focused mechanism of action may mitigate some of these concerns.
It is important to work through the fine shades of these therapeutic differences. Consider the following comparisons:
- Selective Action: AMG487 specifically blocks CXCR3-mediated signaling. This targeted approach means that the defensive functions of other chemokine receptors remain intact, potentially reducing side effects.
- Improved Barrier Function: By preserving tight junction proteins, selective inhibitors not only reduce inflammation but also directly help in re-establishing the structural integrity of the BBB.
- Potential for Combination: Combining targeted inhibitors like AMG487 with agents that reduce cGAS-STING/AIM2 activation might offer synergistic benefits, potentially leading to better outcomes than single-agent therapies.
These comparisons serve as a reminder that, despite the daunting challenges inherent in treating ICH, precise and nuanced interventions can offer real hope. Researchers must figure a path that minimizes off-target effects while maximizing therapeutic gains, a balancing act that is both scientifically demanding and clinically essential.
Future Directions: What Lies Ahead for ICH Therapeutics?
While the research sheds light on new therapeutic targets and paves the way for more refined interventions, several questions remain. Future studies will need to address concerns regarding long-term safety, optimal delivery methods, and the potential for combination therapy. Some of the areas ripe for further investigation include:
- Systemic Delivery Methods: Given that AMG487 currently requires direct intracerebroventricular administration due to its limited BBB penetrability, researchers must explore less invasive techniques that would allow for systemic administration while achieving adequate brain concentrations.
- Gender-Specific Responses: As the current study focused on male models to control for hormonal fluctuations, future research should consider how estrogen and other hormones might alter the inflammatory response in female subjects, potentially requiring different dosing or strategies.
- Multi-Target Approaches: Since the neuroinflammatory response in ICH is full of problems and involves multiple cellular pathways, combination therapies that target several cascades concurrently should be explored. For example, pairing CXCR3 inhibitors with agents that stabilize tight junctions or further modulate astrocytic activity could offer enhanced benefits.
- Extended Outcome Measures: While short-term improvements in neurological function and BBB integrity are promising, long-term research is needed to see if these interventions translate to sustained functional recovery and reduced disability.
These future directions are essential as we continue to untangle the confusing bits associated with ICH. Working through the twists and turns of neuroinflammation requires persistence and precise targeting—the kind of approach that this study has helped to pioneer.
Final Thoughts on Managing the Messy Aftermath of ICH
Intracerebral hemorrhage remains one of the most intimidating neurological emergencies, largely due to its complex aftermath. The breakdown of the blood–brain barrier sets off a chain reaction—including inflammatory cascades and endothelial pyroptosis—that leads to further brain injury. However, the study under discussion has shed much-needed light on the hidden complexities of these processes. By focusing on the CXCL10/CXCR3 axis—and its downstream activation of the cGAS-STING and AIM2 pathways—researchers are beginning to piece together a more complete picture of BBB disruption following ICH.
There is now compelling evidence that selective inhibition of CXCR3 using AMG487 not only dampens the inflammatory cascade but also helps preserve the integrity of the BBB. This preservation is critical, as maintaining a functional barrier is essential for reducing brain edema and minimizing neurological deficits. The study’s use of state-of-the-art in vitro and in vivo models demonstrates that precision targeting of key molecules in inflammatory pathways may provide a viable new avenue for treatment.
While challenges remain—in the form of drug delivery issues, potential gender differences, and the inherent complexity of neuroinflammation—the research paves the way for a more nuanced approach to ICH therapy. Multimodal strategies that combine selective receptor inhibition with agents designed to block specific inflammatory cascades have the potential to yield more effective treatments. As we get into the little details of these mechanisms, the hope is that these advances will eventually translate to clinically meaningful improvements for patients afflicted by ICH.
Conclusion: Charting a Path Towards Improved Neurological Outcomes
In summary, the latest findings on the role of astrocyte-derived CXCL10 and the CXCR3/cGAS/STING/AIM2 pathway in ICH offer significant promise for improving patient outcomes. By reducing endothelial pyroptosis and preserving the BBB through targeted inhibition, researchers are developing strategies that could ultimately lessen the nerve-racking impact of ICH. Although many challenges and tricky parts remain—such as ensuring effective systemic delivery and addressing gender-based variations—this research calls for a thoughtful and layered approach to therapy that could transform the management of this devastating condition.
For clinicians, researchers, and healthcare stakeholders alike, these discoveries provide a clear call to action: to continue investigating multi-target therapies that address the tangled issues of ICH and BBB disruption. As we steer through the obstacles and work to piece together the full picture, the future of ICH treatment looks increasingly promising. Ultimately, it is through continued collaboration, precise targeting of inflammatory pathways, and the integration of neuroprotective strategies that we will be able to make significant strides in mitigating the long-term effects of intracerebral hemorrhage.
In this rapidly evolving field, the marriage of molecular biology and clinical insights offers a roadmap to better care. While the road ahead is loaded with issues and full of complicated pieces, each step forward brings us closer to a time when ICH can be managed more effectively and with fewer lasting consequences for patients. It is our hope that future research will build on these findings, creating a holistic and multi-pronged approach that is as dynamic as the neuroinflammatory processes it aims to counteract.
By embracing these developments with an open mind and a critical eye, the medical community can work to answer many of the lingering questions posed by ICH. With continued research, thoughtful discourse, and an unwavering commitment to patient care, there is every reason to believe that new treatment paradigms will emerge—ones that not only address the hidden complexities of endothelial cell injury and BBB disruption but also pave the way for better, more sustainable neurological outcomes in the long run.
Originally Post From https://www.nature.com/articles/s41420-025-02658-8
Read more about this topic at
NINJ1-mediated plasma membrane rupture of pyroptotic …
Pyroptosis in health and disease: mechanisms, regulation …