Understanding the Role of Apixaban in Thrombus Resolution: A Patient’s Journey
The world of cardiovascular care is full of tricky parts and tangled issues, especially when it comes to managing blood clots in and around the heart. Recent research and case studies have shown that specialized therapies like apixaban can resolve stubborn thrombi in the ascending aorta while also affecting related pulmonary vein thrombi. This opinion editorial takes a closer look at one such case, exploring the fine points of treatment strategies, imaging challenges, and the potential impact on clinical practice.
Grasping the Formation of Cardiac Thrombi and Its Confusing Bits
Thrombus formation in cardiac structures is a subject filled with twists and turns. Although most people commonly understand that blood clots form inside blood vessels, the development of thrombi on the heart’s inner walls or in connected vessels like the pulmonary veins can be especially complicated. In the featured case, a 73-year-old male with hypertension displayed evidence of thrombi in the ascending aorta (AAo) and the right upper pulmonary vein (RUPV), drawing attention to the potential connection between thrombi in different regions of the heart.
The traditional view often points to chronic thrombi in the left atrial appendage (LAA) as culprits in embolic events such as acute ischemic stroke (AIS) or acute myocardial infarction (AMI). However, emerging insights suggest that some thrombi may have already been simmering in the background before these events occur. When medical professionals get into the subject, they find that thrombi are not only composed of blood clots but also have other elements like collagen and, at times, calcifications indicative of older, persistent clots. These hidden complexities force clinicians to rethink the origin of embolic events and to search for more precise diagnostic tools.
Using Cardiac Imaging: The Challenging Parts of Identifying Clot Locations
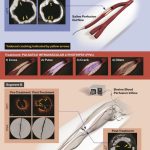
Cardiac computed tomography (CT) and transesophageal echocardiography (TEE) are two widely used methods to detect thrombi. In the case under discussion, TEE revealed highly detailed images suggesting a continuum between RUPV thrombi and AAo thrombi via fine, line-like structures. However, cardiac CT did not have the resolution necessary to pick up all these details; it missed some of the thrombi that TEE highlighted. The contrasting findings between these imaging modalities illustrate the confusing bits in modern diagnostics, where one tool might catch a subtle abnormality while another might have limitations due to timing or resolution.
This gap in detection highlights the need for clinicians to figure a path through a maze of possibilities when diagnosing patients with potential cardiovascular thrombi. The critical takeaway is that combining different imaging techniques might be the best way to get a complete picture of a patient’s thrombus burden. Medical teams must remain flexible and ready to use multiple tools to get around the faults that any single imaging modality might have.
Treatment Strategies: Apixaban Dosage and Its Effects on Thrombus Resolution
One of the most intriguing aspects of the case history is the use of apixaban—a direct oral anticoagulant (DOAC)—to treat complex thrombi in the ascending aorta. Early in the treatment, the patient was given a reduced dose of 2.5 mg twice daily because his weight and other criteria warranted a lower dose for safety. Over the following six months, this regimen proved effective in resolving most of the AAo thrombi, with the imaging studies showing that the previously visible white line-like thrombi connecting the RUPV thrombi to the AAo had largely diminished. This outcome provides hope that a carefully managed anticoagulation strategy can indeed resolve thrombi that had been lingering for quite some time.
However, when the apixaban dose was decreased further—to 2.5 mg only once daily in the subsequent period—the effect was not maintained. Small, regrown thrombi appeared, and the thin connecting lines reappeared. This rebound effect brings forward a central question: how much apixaban is super important to sustain the positive effects, and how delicate is the balance between clot resolution and the risk of clot regrowth?
Understanding these dosing subtleties is key to treating patients safely. Medical professionals must steer through a field of dangerous pitfalls, ensuring that while they are trying to resolve old clots, they are not underdosing and risking regrowth. Therefore, the decision on a specific apixaban dosage should be taken while considering the patient’s weight, kidney function, and the evolving nature of thrombus formation. In short, treatment should be as dynamic as the progression of the clot disease itself.
Analyzing the Imaging Findings: Fine Details of White Thrombi and Line-like Structures
One of the standout elements in the case report is the appearance of what the authors describe as white thrombi and line-like white structures seen running from the RUPV into the AAo. These observations raise important questions about the composition and the behavior of these clots. The presence of white shadows on TEE is particularly interesting because it may indicate calcifications within the thrombi, suggesting a more chronic and organized clot. In contrast, the dark areas found surrounding these white lines could represent regions where ongoing formation or reorganization of clots was taking place.
Clinicians are now forced to consider that these line-like structures might be composed mainly of collagen or organized fibrin bridges. Such features are crucial because they imply that the thrombi are not merely static clogs but active, dynamic structures that could be influenced by changes in medication dosage. In the featured case, when the dose of apixaban was reduced, the reappearance of these line-like thrombi suggests that the process of clot formation did not stop entirely—it merely entered a different phase of activity.
Managing Your Way Through the Treatment Timeline: An Overview
To provide a clearer view of how the treatment evolved over time, it is helpful to examine a table summarizing the dosage adjustments, time frames, and changes in thrombus size alongside key blood test values. The timeline in the presented case allows us to see tangible evidence of how adjustments in the apixaban regimen had both favorable and less-than-optimal outcomes.
| Time Frame | Treatment | Thrombus Size | D-Dimer Level |
|---|---|---|---|
| Start | No treatment | 4 cm × 3 cm | 0.6 μg/ml |
| 6 Months | Apixaban 2.5 mg twice daily | 1 cm × 0.5 cm | 0.5 μg/ml |
| 12 Months | Apixaban 2.5 mg once daily | 2 cm × 1 cm | 0.5 μg/ml |
| 24 Months | Apixaban 2.5 mg once daily | 3 cm × 2 cm | 0.5 μg/ml |
This table underscores how important it is for clinicians and medical teams to “take a closer look” at dosing regimens that might appear to be successful at first glance but could later lead to regrowth of thrombi if not maintained correctly. The small, regrowing thrombi observed during the period of decreased dosage indicate that the resolution of advanced thrombi may require a persistent or tailored approach to dosing—one that does not simply rely on an initial positive response.
The Delicate Balance: Benefits and Risks of Adjusting Apixaban Dosage
Maintaining an optimal apixaban dose is critical to ensuring both efficacy and safety. On one side of the equation, the reduced dose (2.5 mg twice daily) showed promising results in shrinking the thrombi and diminishing the visible connecting white lines between the RUPV and AAo. On the other hand, reducing the dose further or for reasons of preventing side effects can quickly tip the balance, leading to regrowth and potentially setting the stage for future embolic complications.
This scenario presents a nerve-racking situation for physicians who must balance the need to avoid bleeding risks against the risk of thrombus recurrence. If the dose is too high, a patient may experience undesirable side effects; if the dose is too low, the resolution process may not be sustained. The right dosing strategy must be personalized, taking into account factors such as body weight, kidney function, and concurrent cardiovascular issues. In other words, finding your path through these treatment options is both a science and an art, requiring a deep understanding of both the subtle details of clot biology and the patient’s overall health profile.
Exploring the Connection Between Pulmonary Vein and Ascending Aorta Thrombi
Another interesting aspect of this case is the possibility that thrombi in the RUPV might be directly connected to thrombi in the ascending aorta. TEE images showcased a series of white line-like structures that seemed to bridge these two areas. When you take a closer look, it appears that the clot in the pulmonary vein may serve as a starting point or anchor, facilitating the formation of thrombi further downstream in the aorta.
The idea that these thrombi could be linked is both provocative and full of challenges. It forces the medical community to reconsider simple cardiodynamic theories that often attribute complications solely to local factors. Instead, a more integrated perspective is needed—one that considers how a clot in one small section of the heart might have far-reaching consequences. The potential connectivity of thrombi introduces an extra layer of complexity and suggests that more studies are needed to fully understand this phenomenon.
Addressing the Small Distinctions in Clot Composition and Their Clinical Implications
As clinicians dig into the research, they find that not all thrombi are created equal. The white thrombi seen in the RUPV, which appear to have calcified elements and fibrous structures, might be fundamentally different from other clots formed within the heart or the vessels. For instance, unlike the more common blood clots that develop due to stasis or endothelial injury within blood vessels, these white thrombi may embody a more organized structure that involves a variety of cellular elements such as monocytes, macrophages, and even myofibroblasts.
Understanding these fine shades in clot composition is super important. It can help in predicting which patients might be at higher risk for complications like AIS or AMI. Moreover, it reflects that the response to anticoagulants like apixaban could depend heavily on the nature of the clot itself. If the thrombus is highly organized with a rich collagen framework, it might require a more aggressive therapeutic approach or a different management strategy altogether.
Debating the Broader Impact of Thrombus Resolution on Stroke and Heart Attack Prevention
The potential implications of successfully managing AAo and pulmonary vein thrombi reach far beyond the immediate resolution of the clot. Many in the cardiovascular community believe that these thrombi might serve as precursors to serious embolic events such as strokes and heart attacks. Therefore, the ability to partially resolve or even fully eradicate these clots presents a promising opportunity for reducing the risk of such life-threatening complications.
On the flip side, it is equally crucial to analyze whether the act of resolving or modifying these clots with apixaban can inadvertently lead to new issues. For example, could the process of breaking down a large thrombus lead to small emboli that might cause subtle organ damage over time? Although no embolic events were reported in the case over the follow-up period, this possibility remains a question for future research. Until such concerns are addressed, finding your way through treatment decisions will continue to be a process that requires careful monitoring and adjustment.
Examining the Implications for Long-Term Clinical Care and Patient Safety
One of the first questions that arise in any discussion about using apixaban to resolve cardiac thrombi is whether the treatment plan can be safely embedded into long-term clinical practice. This case demonstrates that while apixaban at a certain dose can effectively reduce thrombus size, reducing the dose too much may result in recurrence. In a field that is already loaded with many challenging aspects and potential pitfalls, it seems clear that long-term patient monitoring is key.
Physicians must be prepared to conduct regular imaging studies and blood tests, such as checking D-dimer levels, to ensure that the treatment remains effective over time. It is essential to set up follow-up protocols that allow for timely adjustments to the medication regimen. Such careful patient management helps to steer through the various phases of thrombus resolution and regrowth, ensuring that the therapy continues to offer its protective benefits without incurring additional risk.
Weighing the Pros and Cons: A Closer Look at the Nitty-Gritty of DOAC Therapy
Direct oral anticoagulants (DOACs) like apixaban have rapidly emerged as key players in the management of thrombotic disorders due to their ease of use and stable pharmacokinetics. However, the real-world application of these drugs in complex scenarios—such as the one under discussion—brings several tricky parts to the forefront:
- Effective clot resolution versus thrombus regrowth when modifying doses.
- The potential unseen effects of altering the clot’s structure over time.
- Monitoring for bleeding risks while ensuring sufficient anticoagulation.
- Balancing rapid treatment changes with the slow, often nerve-racking, evolution of thrombus composition.
This balancing act demands that the medical community continually assess their treatment protocols. It is not enough to assume that a one-size-fits-all approach will work for every patient with thrombotic complications. Instead, careful attention must be paid to the individual patient’s response to therapy, the fine shades of clot composition, and the potential for both positive and negative outcomes stemming from changes in dosing.
Patient Safety and Monitoring: Keeping an Eye on the Moving Pieces
The management of thrombi is not just about administering the right dose of medication; it’s also about continuous monitoring. In the case presented, regular TEE evaluations provided crucial evidence of how the patient’s thrombi were reacting to changes in apixaban dosage. Equally important were the blood tests measuring D-dimer levels and kidney function indicators like serum creatinine. These monitoring tools serve as the bedrock for making informed decisions in real time.
For patient safety, it is clear that a regimen needs to incorporate multiple checkpoints: periodic imaging, laboratory studies, and clinical assessments that can detect even slight differences in the patient’s condition. As each piece of data is gathered, physicians can figure a path toward addressing any emerging issues. This proactive approach is key to avoiding the nerve-racking surprises that could lead to severe complications, such as AIS or AMI, which would not only affect patients’ lives but also contribute to the overall burden on healthcare systems.
Learning from the Experience: Practical Takeaways for Clinicians
Reviewing this case offers several practical lessons for clinicians who manage cardiovascular patients with thrombotic complications:
- Precision in Dosing: The experience with apixaban shows how even slight adjustments in dose—tweaked based on patient weight and evolving thrombus characteristics—can have dramatic effects on both success and recurrence rates.
- Importance of Imaging: Relying on just one imaging method may not be sufficient. A combination of TEE and CT can provide a more comprehensive view of thrombus morphology and location. This dual approach can help uncover the small distinctions that might otherwise go unnoticed.
- Constant Reassessment: Thrombus resolution is an ongoing process. Regular monitoring is essential to catch any signs of clot regrowth and to adjust treatment before a potential embolic event occurs.
- Tailored Treatment Protocols: Not every patient will need the same therapeutic approach. Determining the right balance between efficacy and safety requires a personalized strategy, one that is flexible enough to accommodate the shifting landscape of clot formation and resolution.
These bullet points underscore that clinical practice is not static. Instead, it is characterized by constant, sometimes nerve-racking, adjustments based on real-time data and individual patient needs.
Future Directions: What Researchers and Clinicians Need to Poke Around In Next
While this particular case provides valuable insights, it is only one instance in a field that is full of problems and loaded with challenges. There is a clear need for further studies that focus on:
- The Molecular Composition of Thrombi: Analyzing the fine details of thrombus composition—such as the roles of collagen, calcifications, and cellular elements like macrophages and myofibroblasts—could shed light on why some clots respond better to treatment than others.
- Optimizing Apixaban Dosing: Future research must aim to determine the most effective dosing regimen that can maintain thrombus resolution over the long term while minimizing risks. Comparative studies that analyze different dosing schedules could help pinpoint the ideal balance.
- Long-Term Clinical Outcomes: More extensive follow-up studies are necessary to truly understand the impact of thrombus resolution on reducing the risk of AIS, AMI, and other embolic events. Understanding the long-term benefits or drawbacks of DOAC therapy in these contexts is a must-have piece of evidence for shaping future guidelines.
- Advanced Imaging Techniques: As technology improves, newer imaging modalities or enhancements to existing ones could significantly improve detection, characterization, and monitoring of cardiac thrombi. Pioneering these techniques will be essential for managing what can be a tense clinical situation.
By taking a closer look at these research avenues, clinicians and researchers can hope to steer through the confluence of clinical experience and scientific innovation—a journey that may ultimately lead to safer, more effective care for patients battling thrombotic disorders.
Concluding Thoughts: Balancing Innovation with Patient Care
The story of this patient’s experience with apixaban therapy offers a vivid example of the many tricky parts involved in modern cardiology. From the detailed observation of white thrombi and their line-like connections, to the challenges of precise imaging and dynamic dosing—each step in the process is full of nuances and potential pitfalls. Yet, this case also serves as a beacon of hope, reminding clinicians that with careful monitoring, thoughtful dosing, and a willingness to adjust strategies as needed, significant improvements in patient care are possible.
In a field where every small twist and turn can have deep clinical ramifications, the ability to manage your way through the maze of treatment options is as much an art as it is a science. The delicate balance achieved with apixaban in this case exemplifies both the promise and the challenges of current therapeutic approaches. It teaches us that understanding the fine points of clot composition and the dynamics of clot formation can make all the difference in preventing life-threatening events like stroke and heart attack.
Key Points for Clinicians and Healthcare Providers
To summarize some of the essential lessons gleaned from this case, here are several key points to consider:
- Dose Management: A reduced dose of apixaban (2.5 mg twice daily) was effective initially, but subsequent lowering of the dose (2.5 mg once daily) led to regrowth of thrombi. Consistency in dosing may be critical for sustaining benefits.
- Imaging Limitations: While TEE offers detailed images of thrombus morphology, cardiac CT may miss subtle details. A combination of imaging techniques is recommended for a thorough assessment.
- Thrombus Connectivity: The potential bridge between pulmonary vein thrombi and ascending aorta thrombi highlights that clots may not be confined to single regions, complicating treatment approaches.
- Continuous Monitoring: Regular follow-ups using both imaging and laboratory tests such as D-dimer levels are essential for detecting early signs of clot regrowth and adjusting treatment promptly.
- Individualized Therapy: Due to the small distinctions in patients’ responses, treatment plans should be tailored to each patient rather than following a monolithic protocol.
These points reflect the need to be adaptive and proactive in managing thrombotic disorders. Healthcare providers are encouraged to maintain a dialogue with patients about the potential changes in therapy and the importance of regular monitoring to ensure ongoing safety and efficacy.
Final Reflections: The Path Ahead in Managing Cardiac Thrombi
In conclusion, while the case under discussion reveals both positive outcomes and concerning challenges in using apixaban for thrombus resolution, it also opens up numerous questions and research directions for the future. It is clear that chronic thrombi in the heart and proximal vessels are not static objects, but rather dynamic entities influenced by varying treatment regimens. The interplay between the white, seemingly organized thrombi in the pulmonary veins and the ascending aorta echoes broader themes in cardiovascular medicine: the necessity to balance aggressive treatment with ongoing safety, the importance of high-resolution imaging to uncover subtle details, and the need for individualized patient care.
Given that embolic complications such as acute ischemic stroke or myocardial infarction remain frightening possibilities, clinicians must keep abreast of all emerging data regarding DOAC therapy. This means regularly updating protocols, “getting into” the fine details of each patient’s condition, and remaining vigilant to the nerve-racking possibility of thrombus regrowth if treatment is not continuously optimized.
Looking forward, it is super important for researchers to further explore the molecular and structural characteristics of these thrombi. By examining the little details—whether through advanced imaging techniques or detailed biochemical analysis—medical science can develop more effective strategies for preventing, monitoring, and treating thrombotic complications.
Ultimately, the path ahead may be complicated and loaded with issues, but every piece of evidence, every detailed analysis, and every case study contributes to a broader understanding that, in time, will lead to safer and more effective patient care. In the world of cardiovascular medicine, as in life, finding your way through the confusing bits and subtle twists is a journey worth taking, for the reward is the prospect of healthier hearts and better outcomes for every patient.
Originally Post From https://www.cureus.com/articles/409791-resolution-of-ascending-aorta-and-pulmonary-vein-thrombi-with-apixaban-therapy-a-case-study?score_article=true
Read more about this topic at
Time-dependent ultrastructural changes during venous …
Methodological framework and unified metrics for thrombus …