The FDA Approval of Dordaviprone: A New Beacon of Hope for Aggressive Brain Tumors
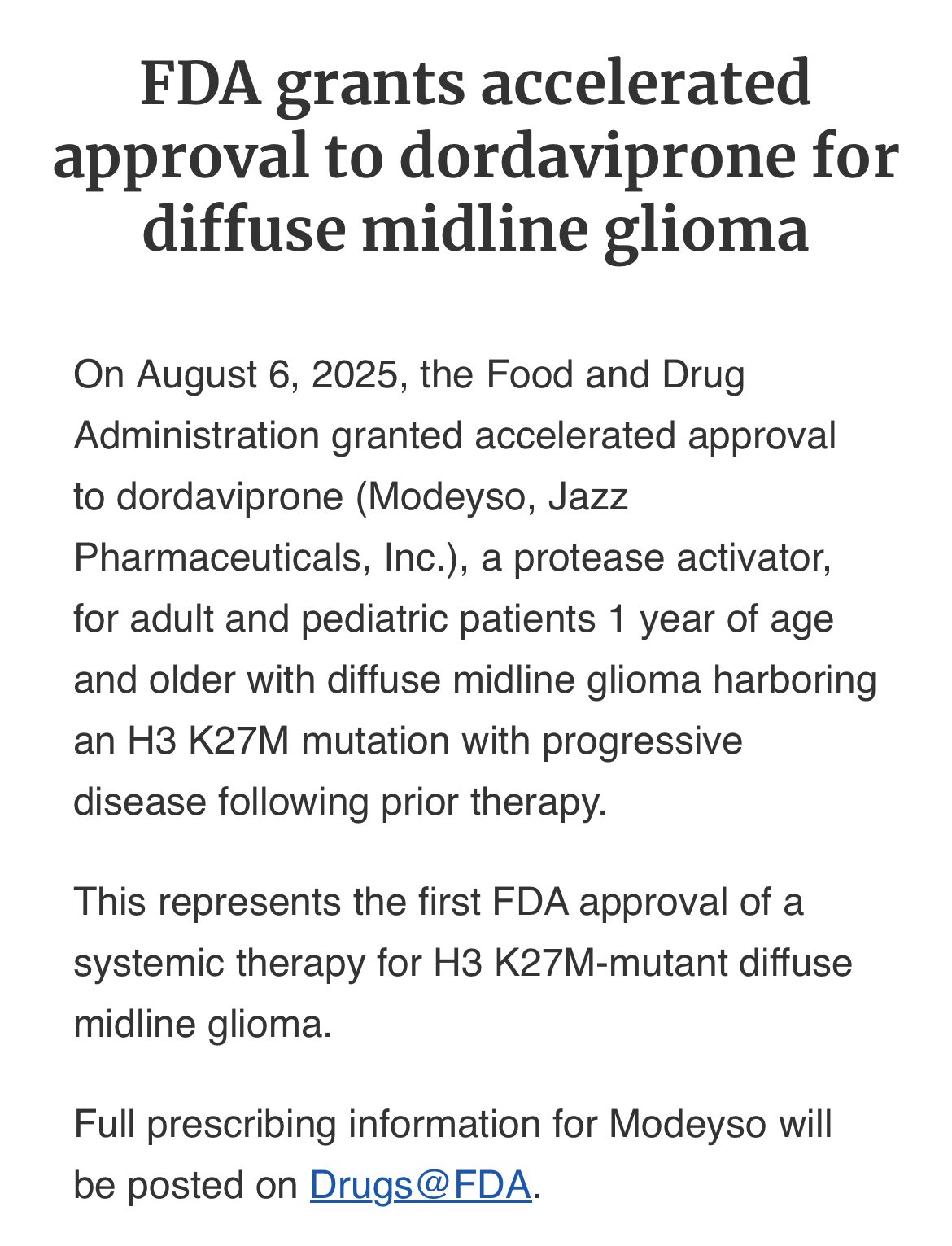
The recent accelerated approval by the FDA for dordaviprone marks a pivotal moment in the fight against one of the most aggressive forms of brain cancer—H3 K27M-mutant diffuse midline glioma (DMG). This approval not only represents a significant advance in targeted treatment options for both pediatric and adult patients, but it also inspires hope in the ongoing battle against a notoriously tricky disease. In this op-ed, we will take a closer look at the developments leading to this landmark decision, examine the clinical data behind it, and explore the wider implications for both patients and the medical community.
Historically, treating DMG has been full of problems, from the overwhelming challenge of surgically accessing the tumor’s location to the twist and turns in response rates seen with conventional therapies. Radiation therapy, once the sole option, offered only a temporary reprieve, as most patients experienced tumor recurrence within a year. With dordaviprone now entering the picture, the landscape is beginning to change. It is a super important milestone because it provides a systemic treatment option that has been shown to be well tolerated, even when previous treatments often left patients feeling overwhelmed by toxic side effects.
Understanding the Unique Challenges in Treating Diffuse Midline Glioma: Tricky Parts and Tangled Issues
Treating diffuse midline glioma isn’t as straightforward as it may first appear. The disease is riddled with tension because these tumors grow quickly, spread unpredictably, and are notoriously difficult to remove surgically. The tumor’s location in the midline of the brain means that surgery is usually limited to biopsies rather than full resections. This confined location makes the tumors not only intimidating but also complicated pieces of medical puzzle that require innovative approaches.
One of the confusing bits in treating H3 K27M-mutant DMG is the molecular driver behind the disease. The mutation causes a failure of normal methylation processes, altering gene expression. This twist in the disease’s biology is one of the hidden complexities that scientists and clinicians have been trying to figure a path through for years. Because of this, traditional treatments that might work for other types of tumors are often off-putting in their inefficacy against DMG. The approval of dordaviprone is significant because it represents a targeted strategy aimed directly at this problematic mutation, a method that ultimately could improve patient survival outcomes.
Evaluating the Efficacy of Dordaviprone in H3 K27M-Mutant Diffuse Midline Gliomas for Both Pediatric and Adult Patients
The approval of dordaviprone was based on an integrated analysis from five open-label clinical trials involving 50 patients with measurable disease. The overall response rate (ORR) of 22% may seem modest at first glance, but when considering the median duration of response (DOR) of over 10 months, a picture of clinical significance emerges. For a disease where patients face a median overall survival of less than one year, these numbers represent a key stepping stone toward improved prognosis.
Let’s break down some of the trial data in a simple table to help illuminate the evidence supporting dordaviprone’s approval:
| Parameter | Value |
|---|---|
| Overall Response Rate (ORR) | 22% |
| Median Duration of Response (DOR) | 10.3 months |
| Patient Population | Both pediatric (1+ year old) and adult patients |
| Study Design | Integrated analysis from 5 open-label clinical trials |
While the response rate may appear low during a quick glance, many experts note that the durability of the response is what makes dordaviprone stand apart. Once a response is achieved, many patients enjoy several more months of progression-free disease, which can potentially allow for combinations with other treatments or serve as a bridge to further research in this area.
How the FDA Accelerated Approval Process Finds a Path for New Glioma Treatments
The FDA’s accelerated approval process is designed to get promising treatments to patients sooner when the need is urgent. In the case of dordaviprone, this approach means that while the approval is based on early efficacy signals, confirmatory trials are still underway to further validate its benefits. The accelerated approval is not the finish line but rather a starting point for further clinical research.
According to experts, one of the trickiest parts of evaluating treatments for DMG is that conventional metrics, such as progression-free survival, can be hard to rely on in a disease with such a rapid pace. Instead, the FDA worked with investigators to accept response rate data (using updated response criteria like RANO 2.0) as a meaningful endpoint. This collaborative approach demonstrates a willingness to steer through the tangled issues that have historically held back progress in treating these tumors.
This strategy is especially important in diseases where time is of the essence. For many patients with H3 K27M-mutant gliomas, waiting for a new treatment to pass traditional clinical trial benchmarks can be literally a matter of life or death. The expedited review process ensures that treatments demonstrating a clear benefit, even if based on early data, can reach patients who are in urgent need.
Dordaviprone’s Tolerability and Safety Profile: A Breath of Fresh Air for Patients
Chemotherapy and targeted drugs for cancer are often loaded with side effects that can be as intimidating as the disease itself. Many of those treatments come with a host of toxicities—fatigue, nausea, and more—that may deter patients from adhering to a treatment regimen. In contrast, dordaviprone is noted for its mild side-effect profile. Patients take the drug only once per week, and the reported side effects, such as headaches, nausea, and a bit of fatigue, tend to be less severe compared to conventional therapies.
This improved tolerability is not just a convenience; it is super important in maintaining a patient’s quality of life during treatment. When a therapy is easier to handle, patients might be more likely to stick with it, thereby potentially leading to better long-term outcomes. Given that many previous treatments have been nerve-racking not only for the physical effects but also for the emotional toll they take, dordaviprone offers a new way to manage the disease that is less taxing on the patient’s overall well-being.
Pioneering New Avenues: Combining Dordaviprone with Other Emerging Therapies
Even though dordaviprone offers some promise as a stand-alone treatment, the real excitement in the oncology community lies in its potential use as part of combination therapies. Experts are eager to see whether pairing dordaviprone with other agents—such as CAR T-cell therapies or novel chemotherapeutics—could amplify its effectiveness. Combining treatments might help overcome the little details that make each individual therapy only partially effective on its own.
Recent studies already suggest that synergistic approaches could pave the way for extended survival and improved response rates in these patients. The current phase 3 ACTION study is expected to offer further insights into how dordaviprone performs in a broader, more controlled setting after radiotherapy, especially in patients with newly diagnosed tumors.
Here are some potential benefits of combination therapies involving dordaviprone:
- Enhanced Response Rates: Working together with other treatments could amplify tumor shrinking effects.
- Extended Duration of Response: Combination treatments might prolong the period of stability once a response is achieved.
- Targeting Multiple Pathways: Addressing several of the disease’s tricky parts at once could yield better outcomes.
- Improved Tolerability: By splitting the therapeutic load between agents, the side effects might be minimized.
These possible avenues illustrate that while dordaviprone alone is a key development, the future could hold even more promising treatment regimens by integrating this drug into multi-modal approaches.
Patient Impact: How a New Treatment Provides Hope Amidst Overwhelming Odds
For the families affected by diffuse midline glioma, every new development represents more than just clinical progress—it symbolizes renewed hope. This disease is characterized by its intimidating nature, where survival times are short and treatment options have been few and far between. Patients and their loved ones are often left grappling with not only the physical effects of the tumor but also the emotional burden of limited choices.
Introducing dordaviprone as a less toxic, targeted option can make a big difference in day-to-day life for many patients. With fewer and less severe side effects, there is a greater chance that patients can maintain a semblance of normalcy during treatment, allowing them to spend more quality time with their families and engage in daily activities with fewer interruptions.
Moreover, the availability of a new option often spurs advocacy efforts, inspiring hope and prompting caregivers, healthcare providers, and patient support groups to push for further research in these challenging areas. The approval of dordaviprone thus represents not just a clinical advancement but also a societal commitment to finding better solutions for a disease that has long been on edge.
Decoding the Clinical Perspectives: How Experts Assess the Trial Data and What It Means for Future Research
In a recent interview, leading neuro-oncology experts provided crucial insights into the trials that led to the approval of dordaviprone. They acknowledged that while tracking the response rate and evaluating the durability of the treatment is full of tricky parts, the results offer a promising foundation. The experts pointed out several tangled issues in clinical data assessment, including the challenge of measuring progression-free survival reliably in this patient population.
One expert noted that the response criteria have been refined over the years—from the earlier RANO high-grade glioma assessments, which only looked at enhancing tumors, to the newer RANO 2.0 criteria that capture both enhancing and nonenhancing tumors. This shift in approach has been critical in providing a more accurate picture of how patients are truly responding to treatment. Here is a comparison of response assessments:
- Older Criteria (RANO high-grade glioma): Measured only enhancing tumors; response rate around 20%.
- Revised Criteria (RANO low-grade glioma): Included nonenhancing tumors; response rate increased to approximately 26%.
- Combined Analysis (RANO 2.0): When both aspects are considered, the response rate approached 30% in some subsets.
This nuanced approach illustrates that while the numbers might seem modest, the refined measurements help ensure that every little twist in the data is accounted for. It also signals that the FDA’s approach in this case was not to rush the process but to base their decision on a careful evaluation of both efficacy and tolerability—making the approval both robust and forward-thinking.
Assessing the Broader Implications on Neurology and Oncology Practice
The approval of dordaviprone is more than a win for patients with H3 K27M-mutant DMG—it also sets a precedent for how treatments for other aggressive tumors might be evaluated in the future. By approving a drug based on integrated data from multiple trials and using evolving response criteria, the FDA is effectively refining how new therapies are judged in conditions with many tricky parts. This approach can be reviewed and potentially replicated in other areas where traditional endpoints fall short or where waiting for long-term survival data may be too nerve-racking for patients in need.
For practicing clinicians, this development simplifies some of the complicated pieces of treatment decision-making. No longer must doctors rely solely on invasive procedures or radiation to manage these tumors; instead, there is now an option that targets the disease more directly while also offering a better side effect profile. This not only helps in reducing the physical burden on patients but also alleviates some of the emotional distress associated with traditional treatment regimens.
Moreover, improvements in response criteria and assessment methods may encourage ongoing dialogue between regulatory agencies and clinical researchers. This cooperative environment could eventually lead to more precise and patient-friendly approval processes for future drugs—especially in fields where treatment outcomes have historically been unpredictable and patient quality of life has been compromised.
Future Directions: Combining Therapies and the Promise of Ongoing Clinical Trials
While dordaviprone’s arrival on the scene is cause for optimism, experts acknowledge that it is just one part of a much larger puzzle. The next line of research is likely to focus on combination therapies and further assessments of clinical benefit. With an ongoing phase 3 ACTION study evaluating the drug’s role after radiotherapy in newly diagnosed patients, the medical community is eager to see if the initial promising signals can be confirmed in a larger, more controlled environment.
Some key areas of future research include:
- Combination with Immunotherapies: Investigating if pairing dordaviprone with emerging immunotherapy techniques, such as CAR T-cell therapy, can enhance the overall response and prolong survival.
- Optimizing Dosage Schedules: Studying whether adjusting the once-weekly regimen based on patient-specific factors could further mitigate side effects and boost efficacy.
- Exploring Expanded Indications: Determining whether the drug’s success in treating thalamic gliomas in young adults might translate to benefits in other hard-to-treat tumors, such as diffuse intrinsic pontine glioma or spinal cord tumors.
- Refining Response Criteria: Continuing to improve and fine-tune the assessment methods to capture subtle details in treatment response more accurately.
These avenues are not just academic—they represent real steps toward a future where even the most intimidating cancers have a suite of effective therapies. While the challenge is still overwhelming, the progress made with dordaviprone is a testament to the potential of combining patient-focused research with innovative clinical strategies.
Lessons Learned from the Development of Dordaviprone: Digging Into the Fine Points of Drug Approval
One of the biggest takeaways from the journey of dordaviprone to FDA approval is that every step of the process must account for the little details that can drastically change patient outcomes. From refining response criteria to rethinking how therapies are combined, every aspect of the development process has been shaped by the need for more precise and patient-friendly approaches.
Some of the main lessons include:
- Collaboration is Key: The close collaboration between researchers, clinicians, and the FDA helped to figure a path through many of the tangled issues inherent in studying rare cancers.
- Flexibility in Data Interpretation: By being open to reanalyzing data using updated criteria like RANO 2.0, the community was able to reveal benefits that might have otherwise been missed.
- Patient Tolerability Matters: The focus on developing a treatment that is not only effective but also easy to tolerate is a paradigm shift away from therapies that, while potent, leave patients with a heavy burden of side effects.
- Continuous Improvement: The approval of dordaviprone is not the end of the road but rather a stepping stone for further improvements in treatment protocols and drug development processes.
The progress made so far is encouraging, yet it also underscores the need for ongoing research. Even though this drug offers hope, researchers and clinicians are fully aware that overcoming such a complex disease will require many more incremental advances that build on these early successes.
How the Integration of Real-World Evidence is Shaping the Future of Glioma Treatment
One aspect that deserves a closer look is the role of real-world evidence (RWE) in shaping drug approvals and treatment guidelines. With rare and aggressive cancers like DMG, traditional randomized controlled trials can leave out many of the fine shades of patient experience that occur in day-to-day clinical practice. RWE—gathered from electronic medical records, registries, and observational studies—provides insights into how treatments play out outside of strictly controlled clinical environments.
For treatments like dordaviprone, integrating real-world data can help clinicians better understand which subsets of patients are likely to benefit the most and how best to manage any tricky parts of the therapy during routine use. Here are some benefits of leveraging real-world evidence:
- Improved Patient Selection: Real-world data can help identify patient characteristics or biomarkers that predict a better response to treatment.
- Enhanced Safety Monitoring: Observational data gathered post-approval can highlight side effects that may not have been fully appreciated during clinical trials.
- Tailored Treatment Approaches: Insights from everyday clinical practice support the development of personalized treatment protocols, ensuring that the benefits of a drug can be maximized while minimizing its risks.
This approach is on the cutting edge of modern medicine, reflecting a broader shift toward precision treatment strategies that take into account not just the disease, but also the individual patient’s experience. By combining the rigor of clinical trials with the nuance of real-world data, the medical community can better manage the twists and turns inherent in treating such a daunting disease.
Patient Stories and the Human Side of Clinical Advances
While numbers and data are critical, the human element of this breakthrough cannot be overlooked. Behind every clinical trial and every FDA approval are patients and families who face a nerve-racking journey each day. The introduction of dordaviprone has already started to change lives for families who once had very limited options against a disease that offered little hope.
Many patients appreciate the gentler side-effect profile, finding that a once-weekly treatment leaves them with more energy to cope with everyday challenges. For others, knowing that there is a treatment that specifically targets the problematic H3 K27M mutation brings not only physical relief but also emotional support—a small ray of light in a period that is often overshadowed by uncertainty.
The emotional and psychological impact cannot be understated. In many cases, new treatment options translate into renewed vigor and determination in facing the disease. Support groups and advocacy organizations have lobbied hard not only for faster approvals, but also for funding to help dig into the subtle parts of the disease that are still misunderstood. This human-centered focus is super important in driving the next generation of research and clinical trials.
The Role of Innovative Technologies in Advancing Therapeutic Approaches for Gliomas
Another exciting dimension to consider is the role that innovative technologies play in modern clinical research. Advances in imaging and molecular diagnostics have made it easier to identify subtle differences between tumors. This ability to get into the nitty-gritty of tumor biology has been a major factor in the development of drugs like dordaviprone.
For example, technologies such as high-resolution MRI and digital pathology have allowed clinicians to better understand the fine points of tumor texture and behavior. When combined with genomic testing, these tools provide a comprehensive picture of the disease’s biology. This information is crucial when trying to figure a path through the tangled issues of response measurement and patient selection.
These advances not only improve our ability to diagnose and monitor the disease but also inform treatment decisions at every step. They help transform what was once a nerve-racking, one-size-fits-all approach into a tailored, data-driven strategy that can be continually refined as more is learned about the disease’s biology and behavior.
Concluding Thoughts: Charting a Course Through the Twists and Turns of Glioma Treatment
The FDA’s accelerated approval of dordaviprone for H3 K27M-mutant diffuse midline glioma is a clear signal that progress is being made in the battle against one of the most intimidating forms of brain cancer. While the journey from laboratory bench to patient bedside is full of tricky parts and complex decisions, each incremental advance brings us closer to a future where aggressive brain tumors can be managed more effectively.
The approval stands as a testament to the power of collaboration between researchers, clinicians, and regulatory bodies. It illustrates how a willingness to dive in and address even the tangled issues of response measurement can lead to treatments that are both effective and easier on patients. With the promise of combination therapies, refined diagnostic tools, and real-world evidence bolstering clinical findings, the horizon for treating DMG and other aggressive tumors looks increasingly promising.
For patients and caregivers, this is not just another drug approval—it is a lifeline in a landscape that has been nerve-racking and full of obstacles. As we continue to weed through the hidden complexities of brain cancer treatment, dordaviprone offers a glimmer of hope that better days are ahead. Though challenges remain, every new piece of data and every innovative study fuels the determination to one day have a comprehensive, compassionate, and effective treatment strategy for all those affected by this disease.
In closing, while the road ahead is still under construction and laden with twists and turns, the approval of dordaviprone is a major milestone that reaffirms our commitment to finding better treatments and ultimately, a cure for aggressive brain tumors. The path may be intricate, but with every step forward, we are learning to steer through the challenges and, most importantly, to offer hope where it is needed most.
Originally Post From https://www.targetedonc.com/view/behind-the-fda-approval-of-dordaviprone-a-new-hope-for-glioma
Read more about this topic at
Breakthrough in treatment approach showing promise in …
“We’re aiming for a cure.” Massey and VIMM researchers …